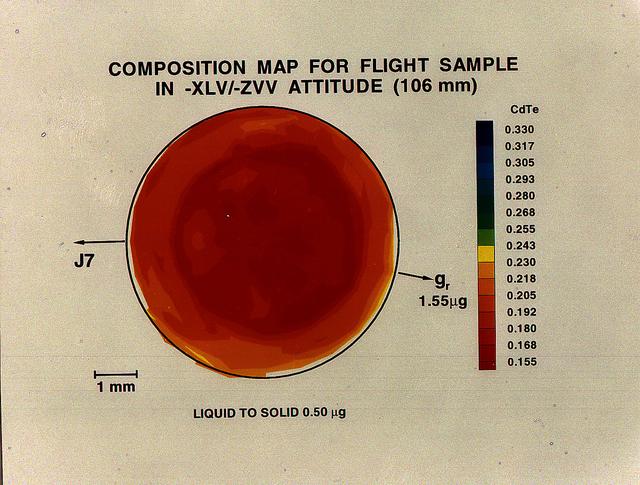
A semiconductor's usefulness is determined by how atoms are ordered within the crystal's underlying three-dimensional structure. While this mercury telluride and cadmium telluride alloy sample mixes completely in Earth -based laboratories, convective flows prevent them from mixing uniformly. In space, the ingredients mix more homogenously, resulting in a superior product.
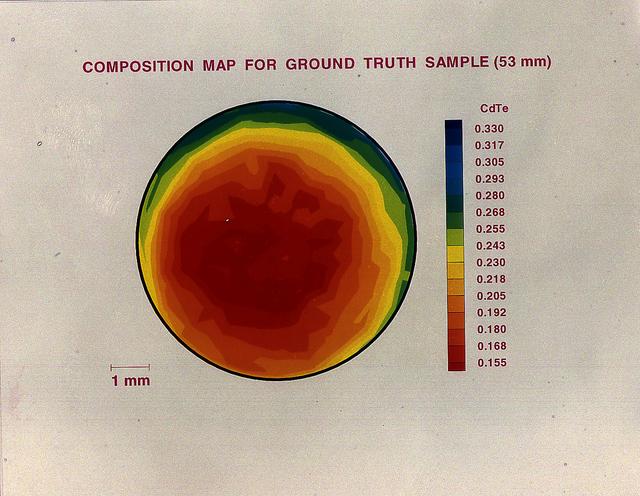
A semiconductor's usefulness is determined by how atoms are ordered within the crystal's underlying three-dimensional structure. While this mercury telluride and cadmium telluride alloy sample mixes completely in Earth -based laboratories, convective flows prevent them from mixing uniformly.
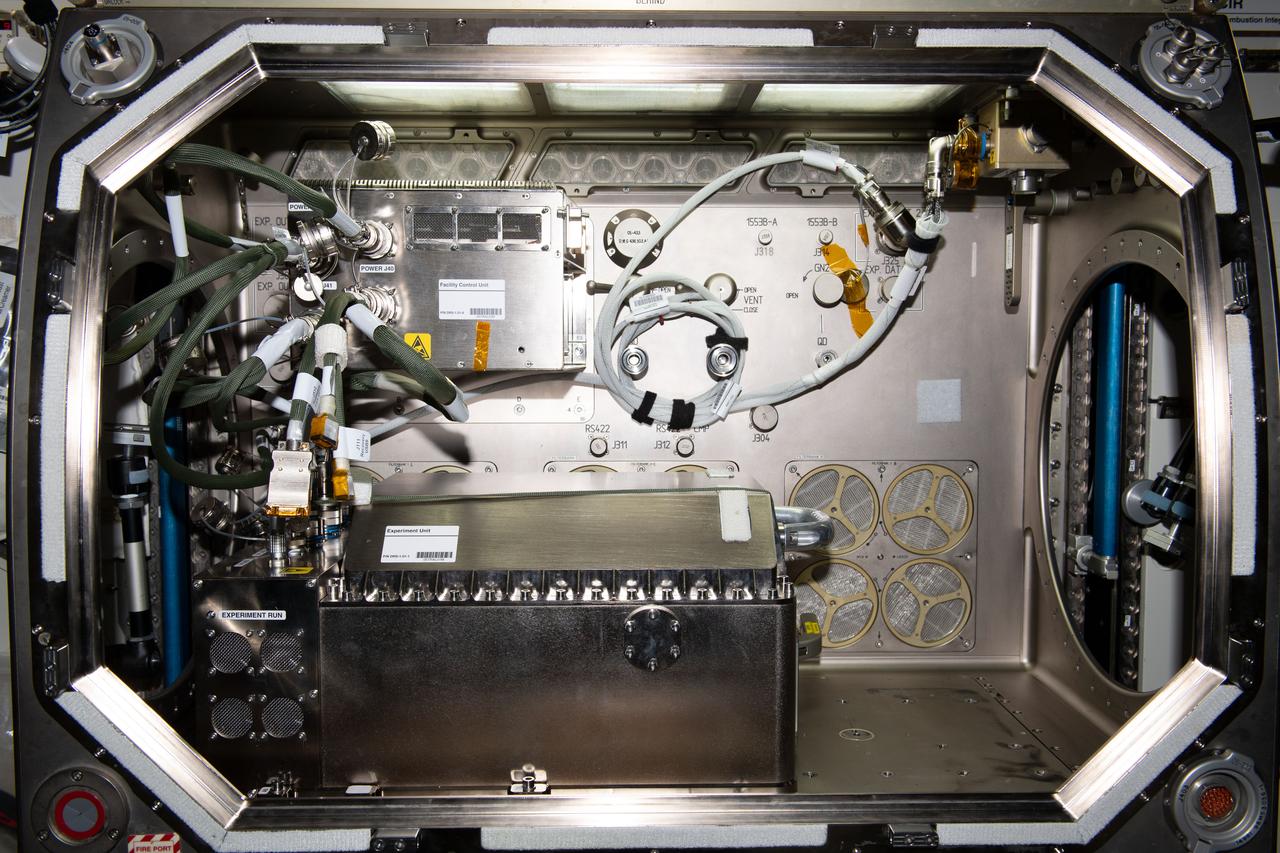
iss070e001580 (10/2/2023) --- A aboard the International Space Station (ISS) of the Transparent Alloys hardware installed into the Microgravity Science Glovebox (MSG) with a sample cartridge. Transparent Alloys consists of numerous experiments to study various growth and solidification processes in alloys.
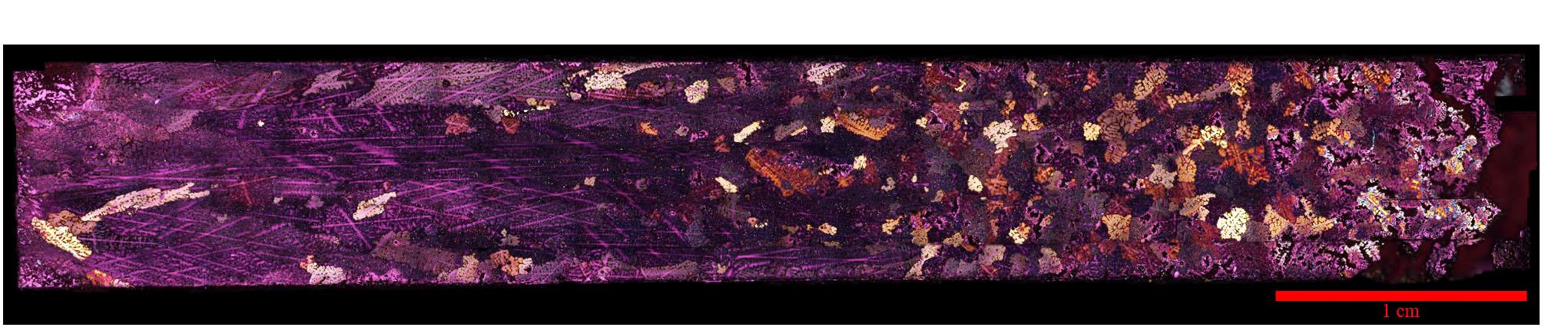
jsc2020e017721 (3/30/2020) --- A preflight view of a Polarized light micrograph of an Al-4%Cu alloy sample solidified in the SUBSA furnace showing a columnar-to-equiaxed transition in the grain structure. The sample has been electrolytically etched to show grains of differing orientation in color contrast under polarized light.
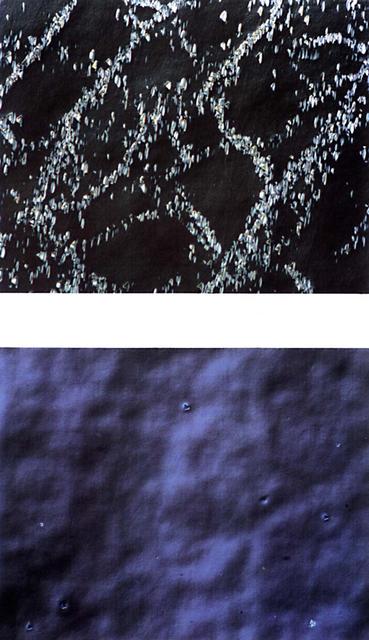
Samples of zinc-alloyed cadmium mercury grown on Earth (1g) and in space (ug) are shown at the same magnification. The space-grown crystal has a more uniform microstructure. Flown on STS-50 USML-1.

jsc2020e017722 (3/30/2020) --- A preflight view of an Alumina ampoule containing an Al-10%Cu alloy sample. The thermocouple wires are secured to the outside of the ampoule with refractory bonding material, while the metal clips provide additional support.

iss038e045758 (2/12/2014) --- A view of Columnar-to-Equiaxed Transition in Solidification Processing-2 (CETSOL-2) test sample 7 which is to be installed into the Material Science Laboratory (MSL) Solidification and Quench Furnace (SQF). This investigation aims to deepen the understanding of the physical principles that govern solidification processes in metal alloys. The patterns of the crystals resulting from transitions of liquids to solids is important for processes used to produce materials such as solar cells, thermoelectrics, and metal alloys.

iss038e045760 92/12/2014) --- A view of Columnar-to-Equiaxed Transition in Solidification Processing-2 (CETSOL-2) test sample 7 which is to be installed into the Material Science Laboratory (MSL) Solidification and Quench Furnace (SQF). This investigation aims to deepen the understanding of the physical principles that govern solidification processes in metal alloys. The patterns of the crystals resulting from transitions of liquids to solids is important for processes used to produce materials such as solar cells, thermoelectrics, and metal alloys.

The purpose of the experiments for the Advanced Automated Directional Solidification Furnace (AADSF) is to determine how gravity-driven convection affects the composition and properties of alloys (mixtures of two or more materials, usually metal). During the USMP-4 mission, the AADSF will solidify crystals of lead tin telluride and mercury cadmium telluride, alloys of compound semiconductor materials used to make infrared detectors and lasers, as experiment samples. Although these materials are used for the same type application their properties and compositional uniformity are affected differently during the solidification process.

jsc2022e072974 (4/15/2022) --- A preflight sample from the Fabrication of Amorphous Metals in Space (MSL SCA-FAMIS) investigation shows tungsten spheres embedded in a glass-forming alloy loaded into a tungsten crucible. Image courtesy of Douglas Hofmann, NASA JPL/Caltech.

ISS012-E-07685 (11 Nov. 2005) --- Astronaut William S. (Bill) McArthur Jr., Expedition 12 commander and NASA space station science officer, photographs Binary Colloidal Alloy Test-3 (BCAT-3) experiment samples in the Destiny laboratory of the international space station.

ISS025-E-008239 (19 Oct. 2010) --- NASA astronaut Shannon Walker, Expedition 25 flight engineer, uses a digital still camera to photograph Binary Colloidal Alloy Test-5 (BCAT-5) experiment samples in the Kibo laboratory of the International Space Station.

KEN COOPER, TEAM LEAD OF MSFC’S ADVANCED MANUFACTURING TEAM, WITH NICKEL ALLOY 718 PARTS FABRICATED USING THE M1 SELECTIVE LASER MELTING SYSTEM. THE M1 MACHINE IS DEDICATED TO BUILDING QUALIFICATION SAMPLES AND HARDWARE DEMONSTRATORS FOR THE RS25 ENGINE PROJECT.

iss066e114140 (Jan. 12, 2022) --- ESA (European Space Agency) astronaut and Expedition 66 Flight Engineer Matthias Maurer swaps samples inside the Materials Science Laboratory, a physics research device that observes metals, alloys, polymers, semiconductors, ceramics, crystals, and glasses, to discover new applications for existing materials and new or improved materials.
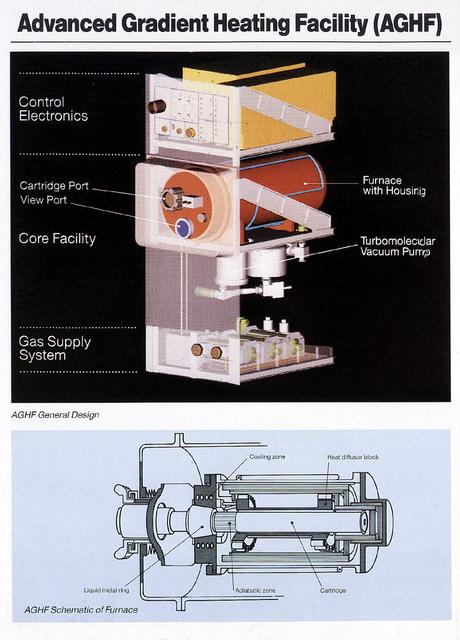
The Advanced Gradient Heating Facility (AGHF) is a European Space Agency (ESA) developed hardware. The AGHF was flown on STS-78, which featured four European PI's and two NASA PI's. The AGHFsupports the production of advanced semiconductor materials and alloys using the directional process, which depends on establishing a hot side and a cold side in the sample.
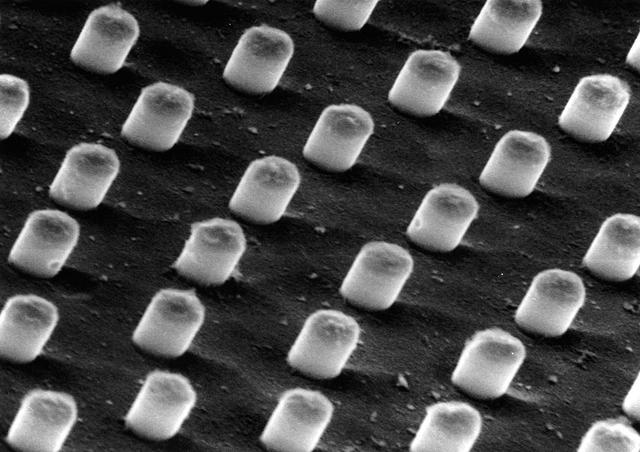
An etched sample of the aluminum indium alloy (magnified). When the hypermonotectic mixture is cooled in the Advanced Gradient Hearing Facility (AGHF), aluminum transitions to a solid first, trapping the indium in cylindrical fibers within the solid. Principal Investigator: Dr. Barry Andrews

iss071e580240 (AUg. 29, 2024) --- Roscosmos cosmonaut and Expedition 71 Commander Oleg Kononenko swaps sample chambers inside the Electromagnetic Levitator (EML) located aboard the International Space Station's Columbus laboratory module. The EML is a physics research device that measures the thermophysical properties of liquid metallic alloys at high temperatures.

iss056e098995 (July 26, 2018) --- Astronaut Alexander Gerst of ESA (European Space Agency) works inside the Japanese Kibo laboratory module taking pictures of samples for the Binary Colloidal Alloy Test-Cohesive Sedimentation investigation (BCAT-CS). The fluid physics research explores the sedimentary properties of quartz and clay particles. Mixed quartz and clay samples are suspended in a liquid for photographic and video downlink to scientists on Earth helping guide future geological studies of unexplored planets and improving petroleum exploration here on Earth.
DR. BINAYAK PANDA LOADS A SAMPLE IN THE IMS-6F SECONDARY ION MASS SPECTROSCOPE’S ULTRA HIGH VACUUM CHAMBER. IT IS CAPABLE OF ANALYZING VERY LIGHT ELEMENTS SUCH AS HYDROGEN AND LITHIUM IN ALLOYS. IT CAN ALSO ANALYZE VERY SMALL QUANTITIES OF IMPURITIES IN MATERIALS AT PARTS PER MILLION LEVELS, AND DETERMINE ISOTOPE RATIOS OF ELEMENTS, ALL IN SOLID SAMPLES.
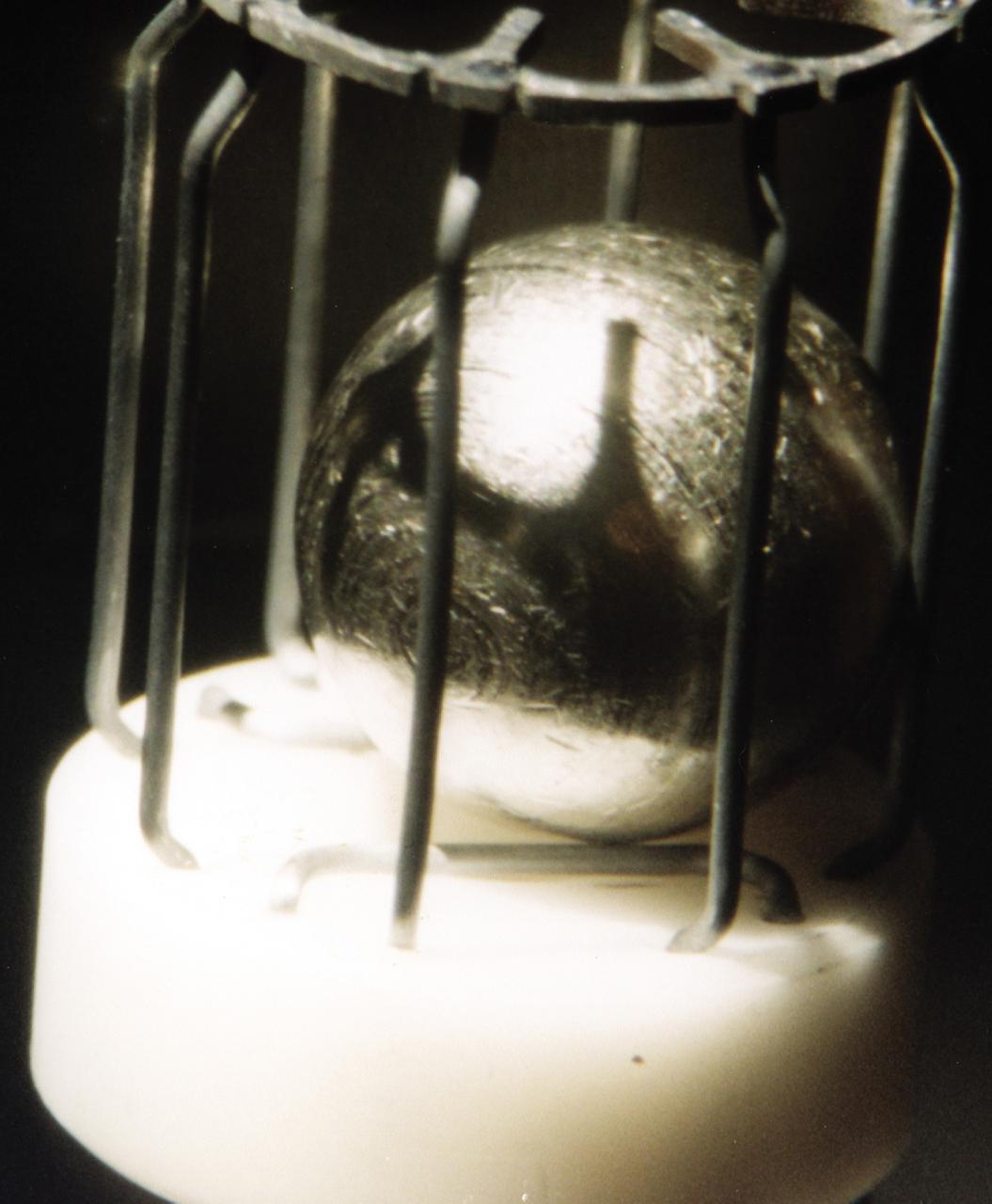
This metal sample, which is approximately 1 cm in diameter, is typical of the metals that were studied using the German designed electromagnetic containerless processing facility. The series of experiments that use this device is known as TEMPUS which is the acronym that stands for the German Tiegelfreies Elektromanetisches Prozessieren Unter Schwerelosigkeit. Most of the TEMPUS experiments focused on various aspects of undercooling liquid metal and alloys. Undercooling is the process of melting a material and then cooling it to a temperature that is below its normal freezing or solidification point. The TEMPUS experiments that used the metal cages as shown in the photograph, often studied bulk metallic glass, a solid material with no crystalline structures. We study metals and alloys not only to build things in space, but to improve things that are made on Earth. Metals and alloys are everywhere around us; in our automobiles, in the engines of aircraft, in our power-plants, and elsewhere. Despite their presence in everyday life, there are many scientific aspects of metals that we do not understand.

Typical metal sample that was processed by TEMPUS (Tiegelfreies Elektromagnetisches Prozessieren Unter Schwerelosigkeit), an electromagnetic levitation facility developed by German researchers and flown on the IML-2 and MSL-1 and 1R Spacelab missions. Electromagnetic levitation is used commonly in ground-based experiments to melt and then cool metallic melts below their freezing points without solidification occurring. Sample size is limited in ground-based experiments. Research with TEMPUS aboard Spacelab allowed scientists to study the viscosity, surface tension, and other properties of several metals and alloys while undercooled (i.e., cooled below their normal solidification points). The sample is about 1 cm (2/5 inch) in diameter.
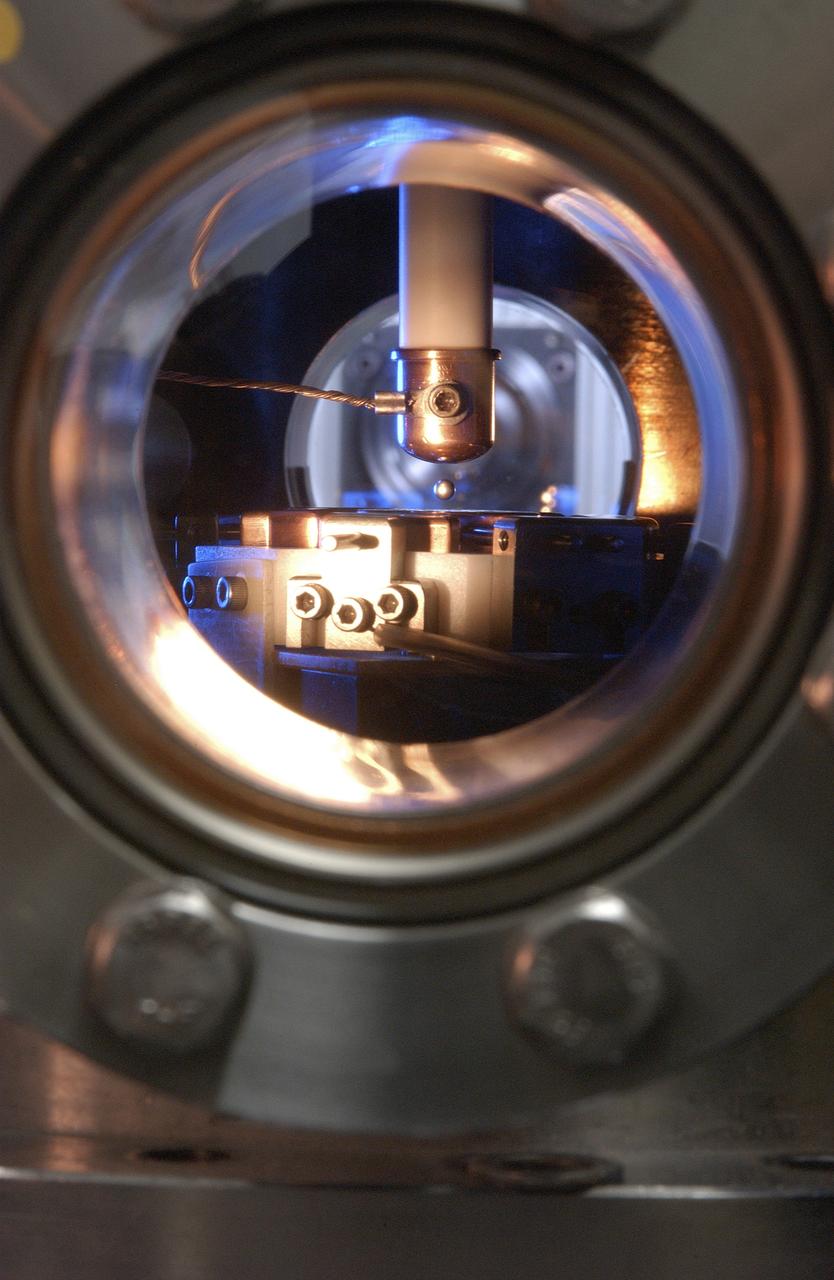
This Photo, which appeared on the July cover of `Physics Today', is of the Electrostatic Levitator (ESL) at NASA's Marshall Space Flight Center (MSFC). The ESL uses static electricity to suspend an object (about 3-4 mm in diameter) inside a vacuum chamber allowing scientists to record a wide range of physical properties without the sample contracting the container or any instruments, conditions that would alter the readings. Once inside the chamber, a laser heats the sample until it melts. The laser is then turned off and the sample cools, changing from a liquid drop to a solid sphere. In this particular shot, the ESL contains a solid metal sample of titanium-zirconium-nickel alloy. Since 1977, the ESL has been used at MSFC to study the characteristics of new metals, ceramics, and glass compounds. Materials created as a result of these tests include new optical materials, special metallic glasses, and spacecraft components.
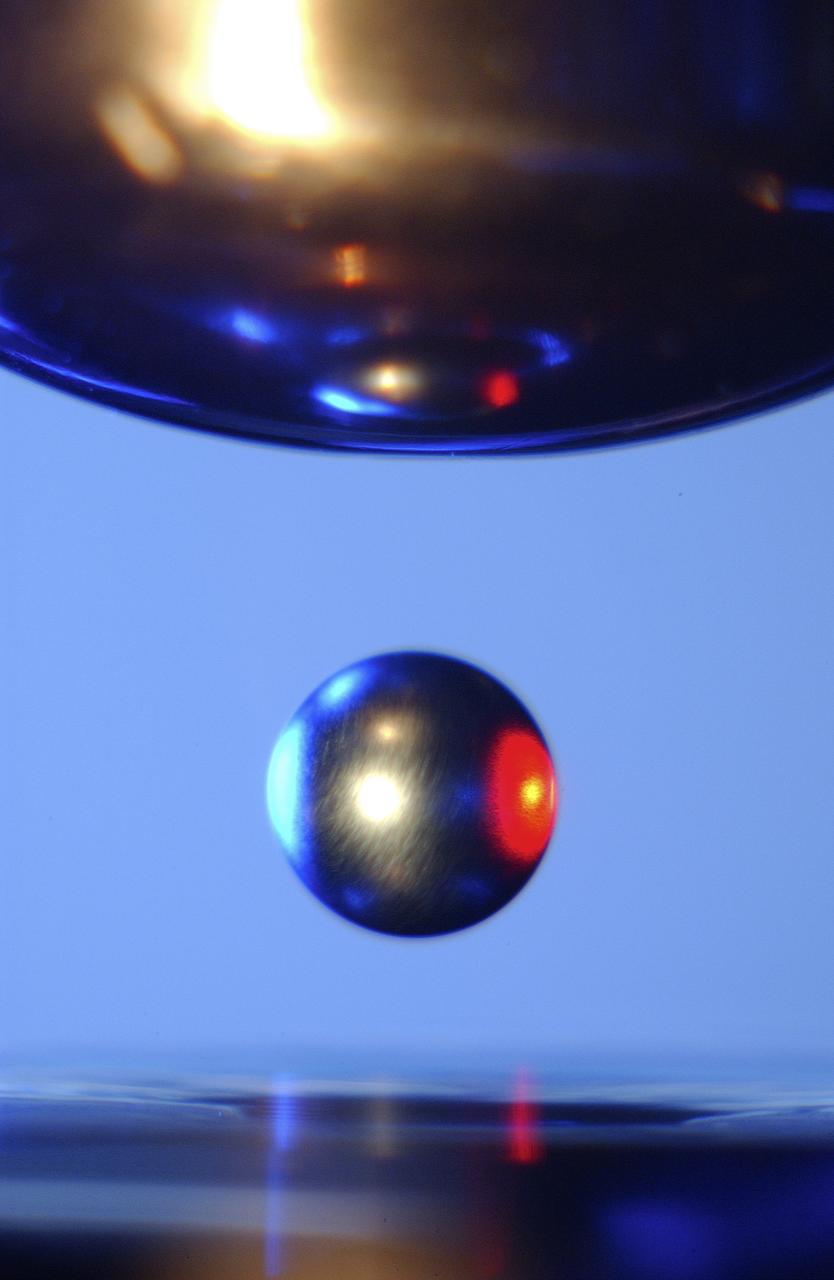
This is a close-up of a sample of titanium-zirconium-nickel alloy inside the Electrostatic Levitator (ESL) vacuum chamber at NASA's Marshall Space Flight Center (MSFC). The ESL uses static electricity to suspend an object (about 3-4 mm in diameter) inside a vacuum chamber allowing scientists to record a wide range of physical properties without the sample contracting the container or any instruments, conditions that would alter the readings. Once inside the chamber, a laser heats the sample until it melts. The laser is then turned off and the sample cools, changing from a liquid drop to a solid sphere. Since 1977, the ESL has been used at MSFC to study the characteristics of new metals, ceramics, and glass compounds. Materials created as a result of these tests include new optical materials, special metallic glasses, and spacecraft components.
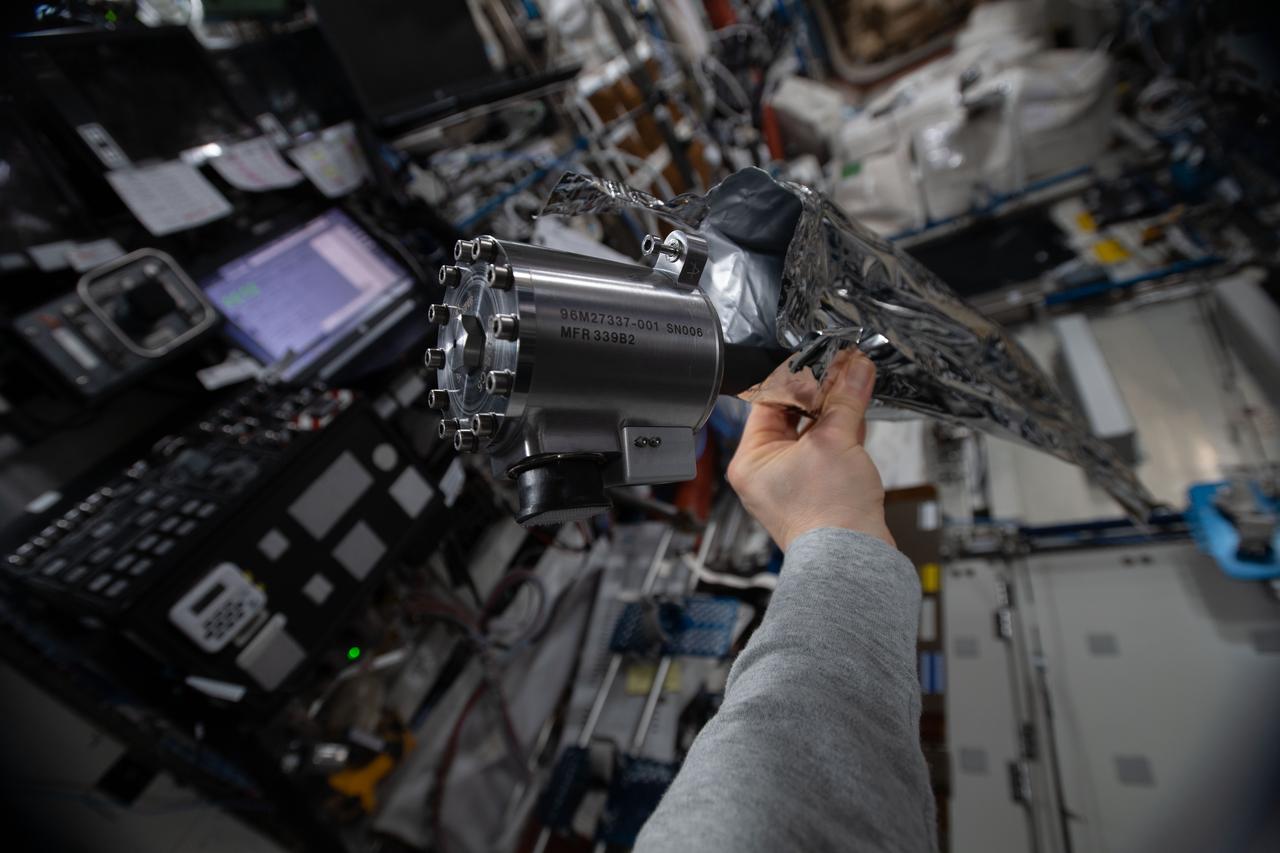
iss061e092274 (12/18/2019) --- A view of the Materials Science Laboratory (MSL) Sample Cartridge Assembly (SCA) in the Destiny module aboard the International Space Station (ISS). The Materials Science Laboratory (MSL) is used for basic materials research in the microgravity environment of the International Space Station (ISS). The MSL can accommodate and support diverse Experiment Modules. In this way many material types, such as metals, alloys, polymers, semiconductors, ceramics, crystals, and glasses, can be studied to discover new applications for existing materials and new or improved materials.

ISS008-E-20613 (5 April 2004) --- Astronaut C. Michael Foale, Expedition 8 commander and NASA ISS science officer, works with a Slow Growth Sample Module (SGSM) for the Binary Colloidal Alloy Test-3 (BCAT) experiment. The SGSM is on a mounting bracket attached to the Maintenance Work Area (MWA) table set up in the Destiny laboratory of the International Space Station (ISS).

iss073e0071487 (May 15, 2025) --- NASA astronaut and Expedition 73 Flight Engineer Nichole Ayers swaps sample cartridges inside the Material Science Laboratory (MSL) that supports high temperature space physics research using furnaces aboard the International Space Station's Destiny laboratory module. The properties of many types of materials such as metals, alloys, polymers, semiconductors, ceramics, crystals, and glasses, can be studied in the MSL to discover new applications for existing materials and new or improved materials.

iss065e081296 (May 28, 2021) --- NASA astronaut and Expedition 65 Flight Engineer Megan McArthur reviews procedures to swap sample cartridges inside the Materials Science Laboratory (MSL). The MSL enables research into microgravity's affects on materials such as metals, alloys, polymers, semiconductors, ceramics, crystals, and glasses. Observations may reveal new applications for existing materials and new or improved materials.

ISS008-E-20610 (5 April 2004) --- Astronaut C. Michael Foale, Expedition 8 commander and NASA ISS science officer, uses a digital still camera to photograph a Slow Growth Sample Module (SGSM) for the Binary Colloidal Alloy Test-3 (BCAT) experiment. The SGSM is on a mounting bracket attached to the Maintenance Work Area (MWA) table set up in the Destiny laboratory of the International Space Station (ISS).

iss056e098988 (July 26, 2018) --- Photographic documentation of the Binary Colloidal Alloy Test-Cohesive Sedimentation investigation (BCAT-CS). The fluid physics research explores the sedimentary properties of quartz and clay particles. Mixed quartz and clay samples are suspended in a liquid for photographic and video downlink to scientists on Earth helping guide future geological studies of unexplored planets and improving petroleum exploration here on Earth.

iss071e522745 (Aug. 19, 2024) --- NASA astronaut and Expedition 71 Flight Engineer Mike Barratt swaps sample cartridges inside the Materials Science Laboratory (MSL), a research furnace facilitating discoveries of new and improved materials as well as new uses for existing materials such as metals, alloys, polymers, and more. The MSL is located inside the International Space Station's Destiny laboratory module.

iss065e081297 (May 28, 2021) --- NASA astronaut and Expedition 65 Flight Engineer Megan McArthur swaps sample cartridges inside the Materials Science Laboratory (MSL) rack. The MSL enables observations of microgravity's impact on a variety metals, alloys, polymers, semiconductors, ceramics, crystals, and glasses, to discover new applications for existing materials and new or improved materials.

ss038e008298 (11/26/2013) --- A view of NASA astronaut Rick Mastracchio, during the Material Science Laboratory (MSL) Solidification and Quench Furnace (SQF) Sample Cartridge Exchange aboard the International Space Station (ISS). The Materials Science Laboratory (MSL) is used for basic materials research in the microgravity environment of the ISS. The MSL can accommodate and support diverse Experiment Modules. In this way many material types, such as metals, alloys, polymers, semiconductors, ceramics, crystals, and glasses, can be studied to discover new applications for existing materials and new or improved materials.
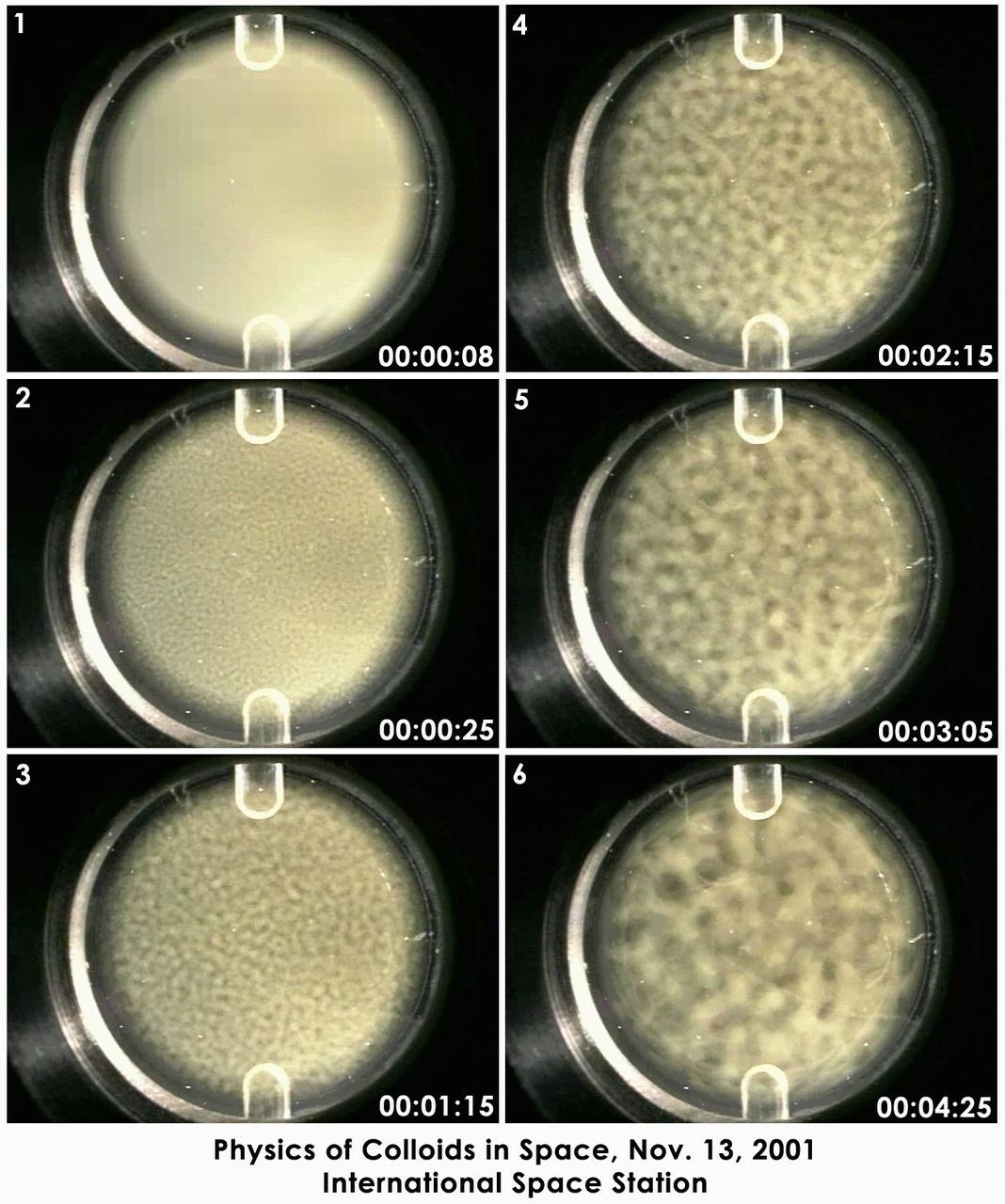
Still photographs taken over 16 hours on Nov. 13, 2001, on the International Space Station have been condensed into a few seconds to show the de-mixing -- or phase separation -- process studied by the Experiment on Physics of Colloids in Space. Commanded from the ground, dozens of similar tests have been conducted since the experiment arrived on ISS in 2000. The sample is a mix of polymethylmethacrylate (PMMA or acrylic) colloids, polystyrene polymers and solvents. The circular area is 2 cm (0.8 in.) in diameter. The phase separation process occurs spontaneously after the sample is mechanically mixed. The evolving lighter regions are rich in colloid and have the structure of a liquid. The dark regions are poor in colloids and have the structure of a gas. This behavior carnot be observed on Earth because gravity causes the particles to fall out of solution faster than the phase separation can occur. While similar to a gas-liquid phase transition, the growth rate observed in this test is different from any atomic gas-liquid or liquid-liquid phase transition ever measured experimentally. Ultimately, the sample separates into colloid-poor and colloid-rich areas, just as oil and vinegar separate. The fundamental science of de-mixing in this colloid-polymer sample is the same found in the annealing of metal alloys and plastic polymer blends. Improving the understanding of this process may lead to improving processing of these materials on Earth.
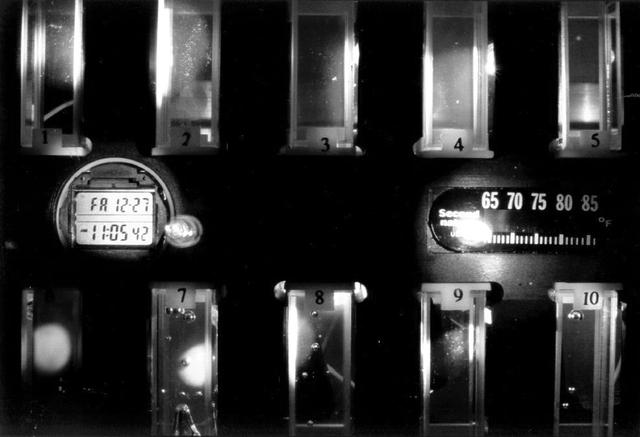
Close-up view of the Binary Colloidal Alloy Test during an experiment run aboard the Russian Mir space station. BCAT is part of an extensive series of experiments plarned to investigate the fundamental properties of colloids so that scientists can make colloids more useful for technological applications. Some of the colloids studied in BCAT are made of two different sized particles (binary colloidal alloys) that are very tiny, uniform plastic spheres. Under the proper conditions, these colloids can arrange themselves in a pattern to form crystals, which may have many unique properties that may form the basis of new classes of light switches, displays, and optical devices that can fuel the evolution of the next generation of computer and communication technologies. This Slow Growth hardware consisted of a 35-mm camera aimed toward a module which contained 10 separate colloid samples. To begin the experiment, one of the astronauts would mix the samples to disperse the colloidal particles. Then the hardware operated autonomously, taking photos of the colloidal samples over a 90-day period. The investigation proved that gravity plays a central role in the formation and stability of these types of colloidal crystal structures. The investigation also helped identify the optimum conditions for the formation of colloidal crystals, which will be used for optimizing future microgravity experiments in the study of colloidal physics. Dr. David Weitz of the University of Pennsylvania and Dr. Peter Pusey of the University of Edinburgh, United Kingdom, are the principal investigators.

Dr. Jennifer Williams, a NASA research chemical engineer, displays two fatigue samples that will be tested in the Plasma Rapid Oxidation Technique for Extending Component Tenability (PROTECT) experiments inside the Prototype Laboratory at NASA’s Kennedy Space Center in Florida on Nov. 2, 2022. Plasma electrolytic oxidation is a surface coating technology that produces oxide layers on the surface of light metals and their alloys to improve their performance characteristics. These coatings are tailored to provide a combination of characteristics such as corrosion protection, wear resistance, thermal management, extreme hardness, and fatigue performance. PROTECT is expected to demonstrate a 10 percent improved fatigue performance and a 70 percent improvement in corrosion characteristics on the interior of treated 3-D printed metallic parts when compared to non-treated parts. PROTECT could be applied to spacecraft and launch vehicles.

Gerard Moscoso, a mechanical engineer technician with NASA, handles a sample that is being prepared for fatigue and corrosion testing for the Plasma Rapid Oxidation Technique for Extending Component Tenability (PROTECT) project inside the Prototype Development Laboratory at NASA’s Kennedy Space Center in Florida on Nov. 2, 2022. Plasma electrolytic oxidation is a surface coating technology that produces oxide layers on the surface of light metals and their alloys to improve their performance characteristics. These coatings are tailored to provide a combination of characteristics such as corrosion protection, wear resistance, thermal management, extreme hardness, and fatigue performance. PROTECT is expected to demonstrate a ten percent improved fatigue performance and a 70 percent improvement in corrosion characteristics on the interior of treated 3-D printed metallic parts when compared to non-treated parts. PROTECT could be applied on spacecraft and launch vehicles.

Gerard Moscoso, a mechanical engineer technician with NASA, prepares a sample for testing for the Plasma Rapid Oxidation Technique for Extending Component Tenability (PROTECT) project inside the Prototype Development Laboratory at NASA’s Kennedy Space Center in Florida on Nov. 2, 2022. Plasma electrolytic oxidation is a surface coating technology that produces oxide layers on the surface of light metals and their alloys to improve their performance characteristics. These coatings are tailored to provide a combination of characteristics such as corrosion protection, wear resistance, thermal management, extreme hardness, and fatigue performance. PROTECT is expected to demonstrate a 10 percent improved fatigue performance and a 70 percent improvement in corrosion characteristics on the interior of treated 3-D printed metallic parts when compared to non-treated parts. PROTECT could be applied on spacecraft and launch vehicles.