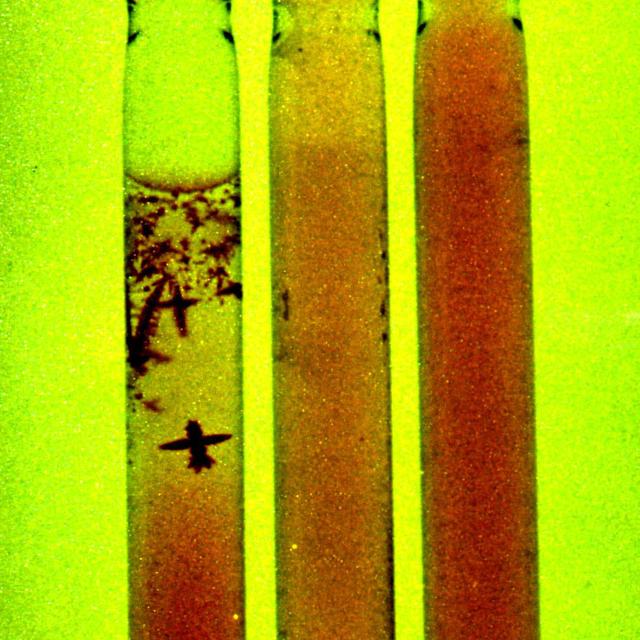
Dr. Alexander Chernov, of the Universities Space Research Association (USRA) and based at Marshall Space Flight Center, is investigating why protein crystals grown in space are, in about 20 percent of cases, better-ordered than those grown on the ground. They are testing the idea that the amount of impurities trapped by space-grown crystals may be different than the amount trapped by crystals grown on Earth because convection is negligible in microgravity. The concentrations or impurities in many space-grown crystals turned out to be several times lower than that in the terrestrial ones, sometimes below the detection limit. The ground-based experiment also showed that the amount of impurities per unit volume of the crystals was usually higher than the amount per unit volume of the solution. This means that a growing crystal actually purifies the solution in its immediate vicinity. Here, an impurity depletion zone is created around apoferritin crystals grown in gel, imitating microgravity conditions.
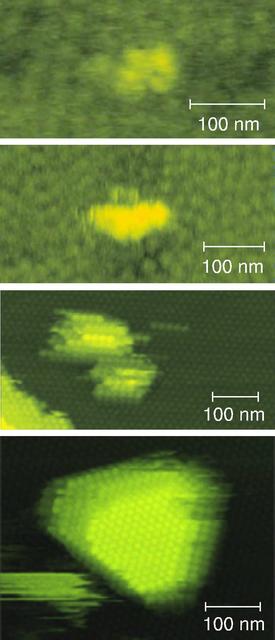
Watching molecules of the iron-storing protein apoferritin come together to form a nucleus reveals some interesting behavior. In this series of images, researchers observed clusters of four molecules at the corners of a diamond shape (top). As more molecules attach to the cluster, they arrange themselves into rods (second from top), and a raft-like configuration of molecules forms the critical nucleus (third from top), suggesting that crystal growth is much slower than it could be were the molecules arranged in a more compact formation. In the final image, a crystallite consisting of three layers containing approximately 60 to 70 molecules each is formed. Atomic force microscopy made visualizing the process of nucleation possible for the first time. The principal investigator is Peter Vekilov, of the University of Alabama in Huntsville. Vekilov's team at UAH studies protein solutions as they change phases from liquids to crystalline solids. They want to know if the molecules in the solution interact with one another, and if so, how, from the perspectives of thermodynamics and kinetics. They want to understand which forces -- electrical, electrostatic, hydrodynamic, or other kinds of forces -- are responsible for the interactions. They also study nucleation, the begirning stage of crystallization. This process is important to understand because it sets the stage for crystal growth in all kinds of solutions and liquid melts that are important in such diverse fields as agriculture, medicine, and the fabrication of metal components. Nucleation can determine the rate of crystal growth, the number of crystals that will be formed, and the quality and size of the crystals.

The demetalized form of the major iron storage protein from horse spleen.