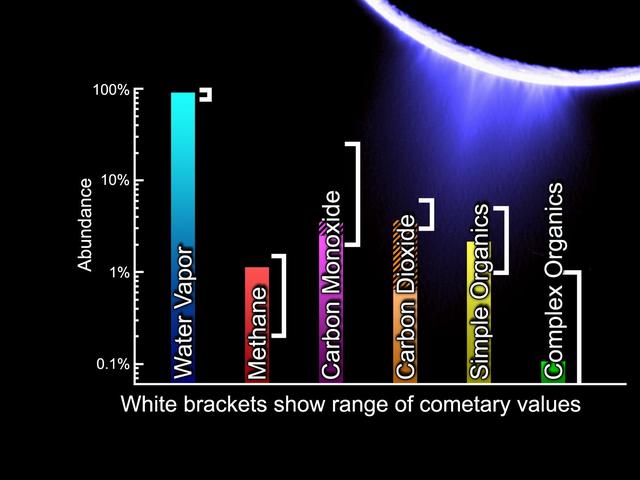
Comets and Enceladus - Similar Chemistry

Delivery to the Wet Chemistry Laboratory
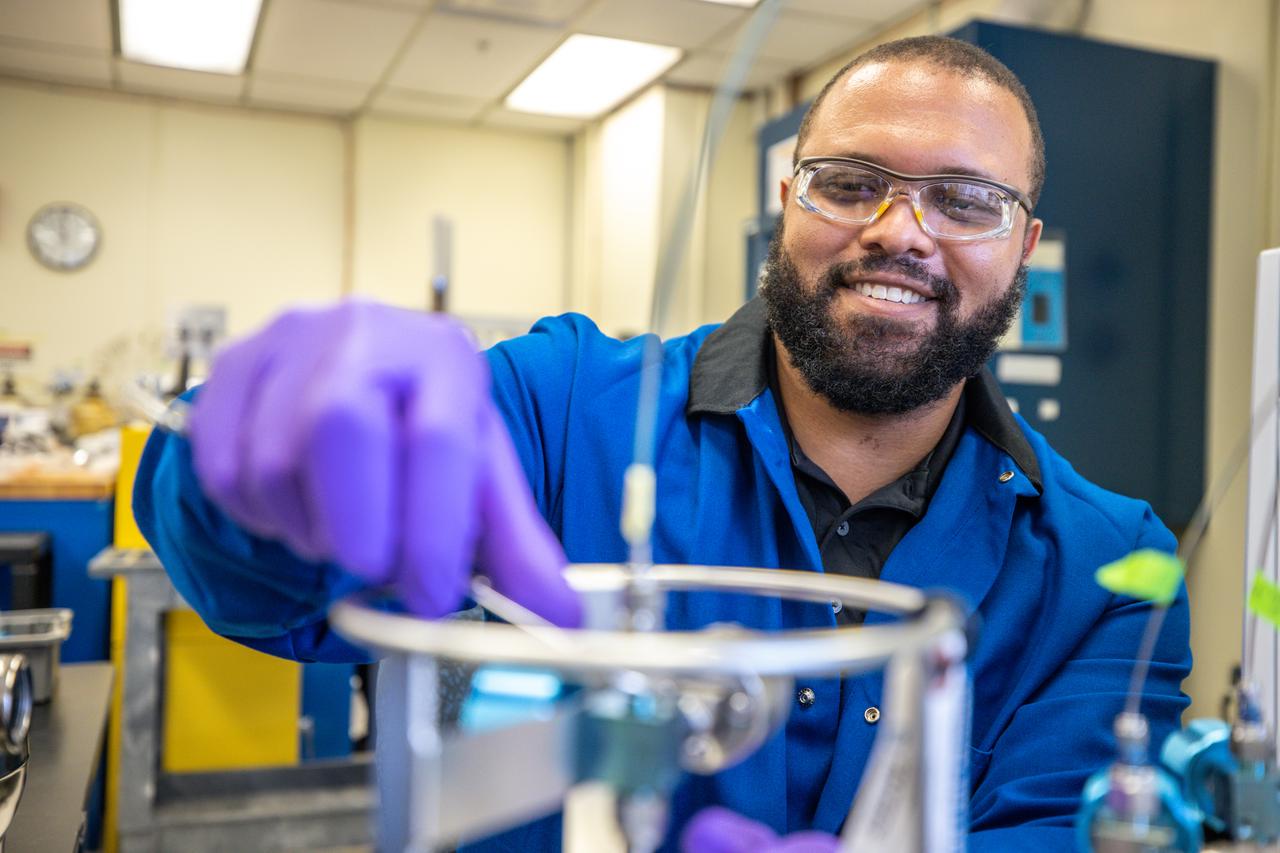
Chemist Trey Barnes prepares a gas sample for injection into a gas chromatography-mass spectrometry system preconcentrator for analyzing trace level gas contaminants inside NASA Engineering’s Analytical Laboratories at Kennedy Space Center in Florida on July 7, 2021. One of seven branches in the NASA Laboratories, Development, and Testing Division, the Analytical Laboratories branch provides microscopic imagery and analysis through the use of a wide variety of microscopic techniques to identify contaminants and other urgent problems associated with aerospace flight hardware, ground support equipment, and related facilities.

Lead chemist Philip Howard poses for a photo inside NASA Engineering’s Analytical Laboratories at Kennedy Space Center in Florida on July 7, 2021. One of seven branches in the NASA Laboratories, Development, and Testing Division, the Analytical Laboratories branch provides microscopic imagery and analysis through the use of a wide variety of microscopic techniques to identify contaminants and other urgent problems associated with aerospace flight hardware, ground support equipment, and related facilities.

Chemists from NASA Engineering’s Analytical Laboratories at Kennedy Space Center in Florida pose for a photo near a scanning electron microscope on July 7, 2021. From left to right is Macy Mullen, structural materials; Athela Frandsen, structural materials; Philip Howard, lead, structural materials; Janelle Coutts, gas and fluid systems; David Rinderknecht, structural materials; Trey Barnes, Earth biosphere studies; and Misle Tessema, Earth biosphere studies. One of seven branches in the NASA Laboratories, Development, and Testing Division, the Analytical Laboratories branch provides microscopic imagery and analysis through the use of a wide variety of microscopic techniques to identify contaminants and other urgent problems associated with aerospace flight hardware, ground support equipment, and related facilities.
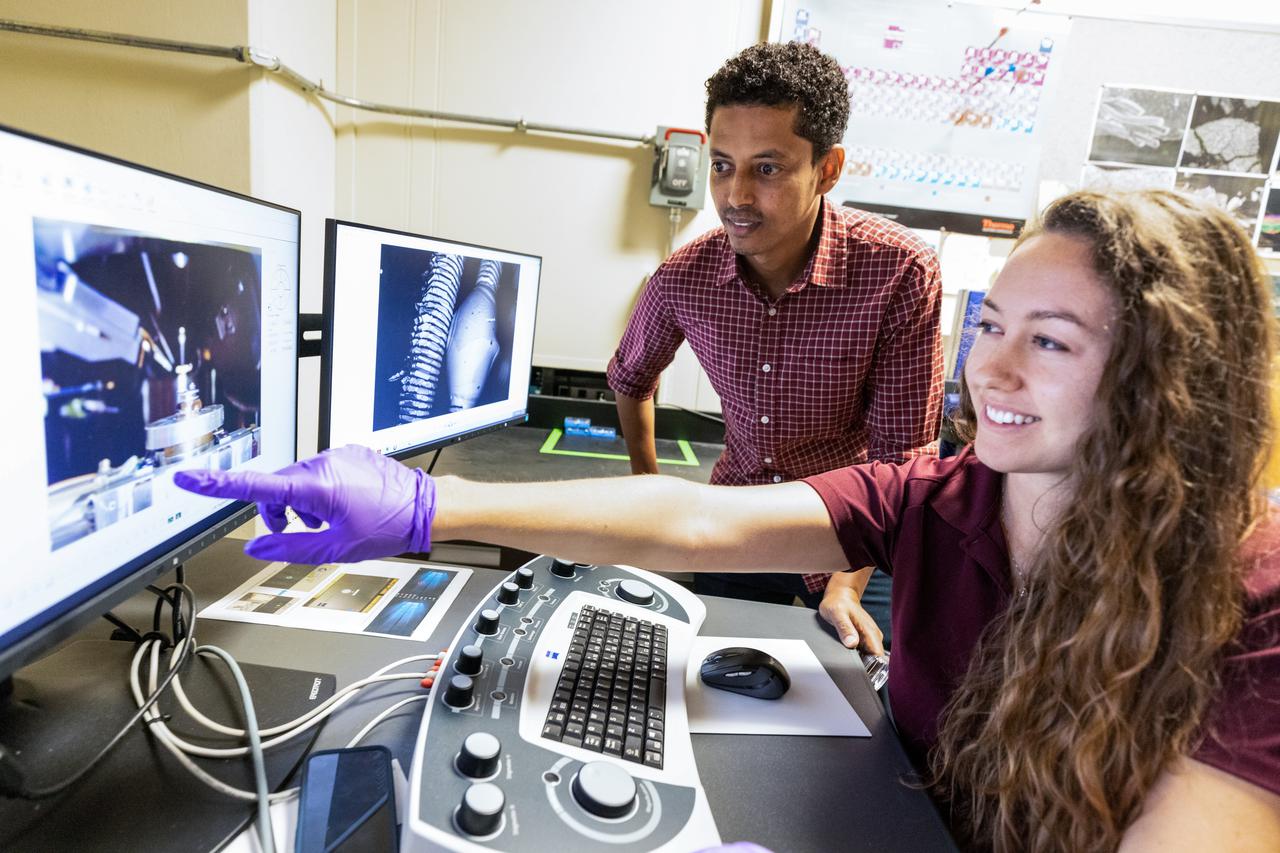
Chemists Misle Tessema (left) and Macy Mullen (right) discuss scanning electron microscope operations inside NASA Engineering’s Analytical Laboratories at Kennedy Space Center in Florida on July 7, 2021. One of seven branches in the NASA Laboratories, Development, and Testing Division, the Analytical Laboratories branch provides microscopic imagery and analysis through the use of a wide variety of microscopic techniques to identify contaminants and other urgent problems associated with aerospace flight hardware, ground support equipment, and related facilities.
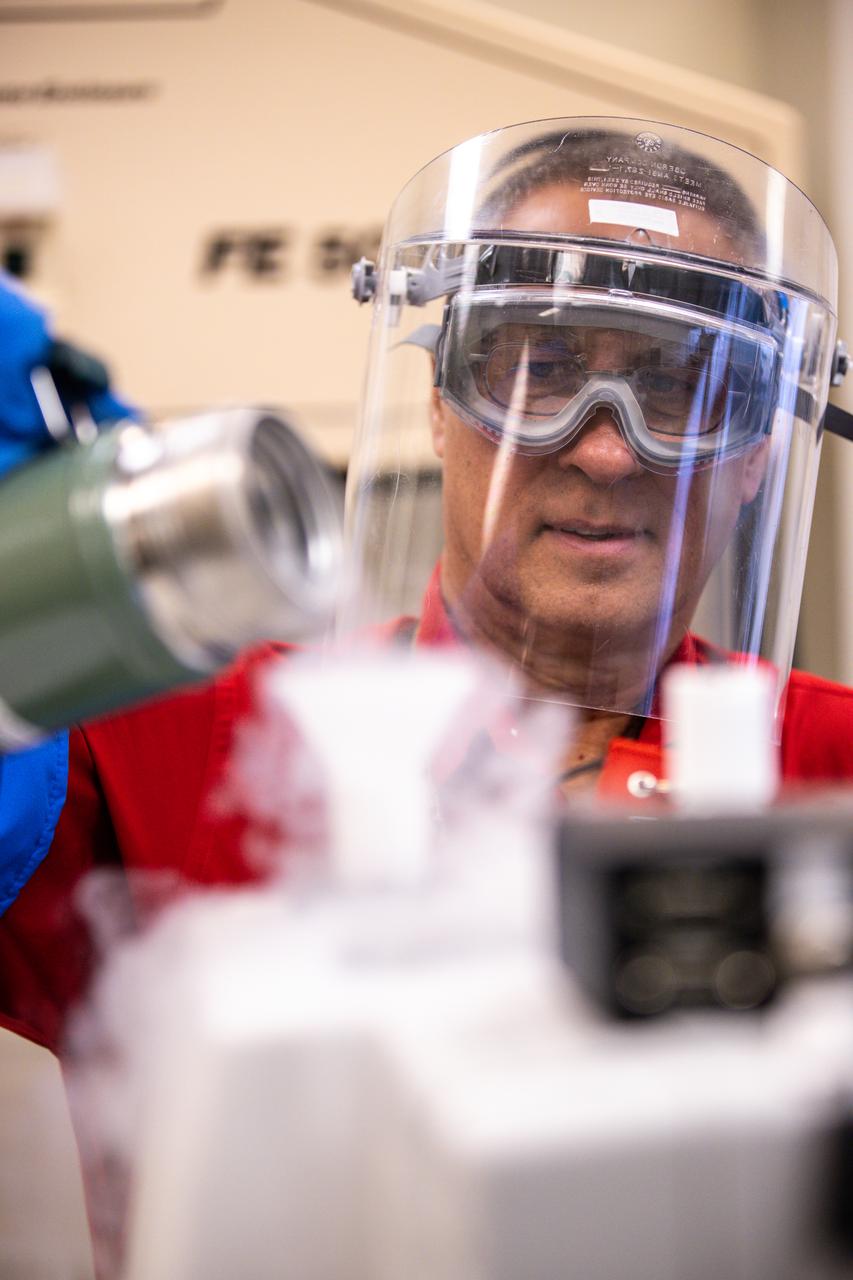
Lead chemist Philip Howard pours liquid nitrogen into the Fourier-transform infrared spectrometer to cool the detector in NASA Engineering’s Analytical Laboratories at Kennedy Space Center in Florida on July 7, 2021. One of seven branches in the NASA Laboratories, Development, and Testing Division, the Analytical Laboratories branch provides microscopic imagery and analysis through the use of a wide variety of microscopic techniques to identify contaminants and other urgent problems associated with aerospace flight hardware, ground support equipment, and related facilities.

Chemist Athela Frandsen from NASA Engineering’s Analytical Laboratories at Kennedy Space Center in Florida loads a sample into a scanning electron microscope on July 7, 2021. One of seven branches in the NASA Laboratories, Development, and Testing Division, the Analytical Laboratories branch provides microscopic imagery and analysis through the use of a wide variety of microscopic techniques to identify contaminants and other urgent problems associated with aerospace flight hardware, ground support equipment, and related facilities.

Chemist David Rinderknecht analyzes a sample on the stereomicroscope inside NASA Engineering’s Analytical Laboratories at Kennedy Space Center in Florida on July 7, 2021. One of seven branches in the NASA Laboratories, Development, and Testing Division, the Analytical Laboratories branch provides microscopic imagery and analysis through the use of a wide variety of microscopic techniques to identify contaminants and other urgent problems associated with aerospace flight hardware, ground support equipment, and related facilities.

Senior analytical chemist Janelle Coutts injects a sample for gas chromatography-mass spectrometry analysis inside NASA Engineering’s Analytical Laboratories at Kennedy Space Center in Florida on July 7, 2021. One of seven branches in the NASA Laboratories, Development, and Testing Division, the Analytical Laboratories branch provides microscopic imagery and analysis through the use of a wide variety of microscopic techniques to identify contaminants and other urgent problems associated with aerospace flight hardware, ground support equipment, and related facilities.
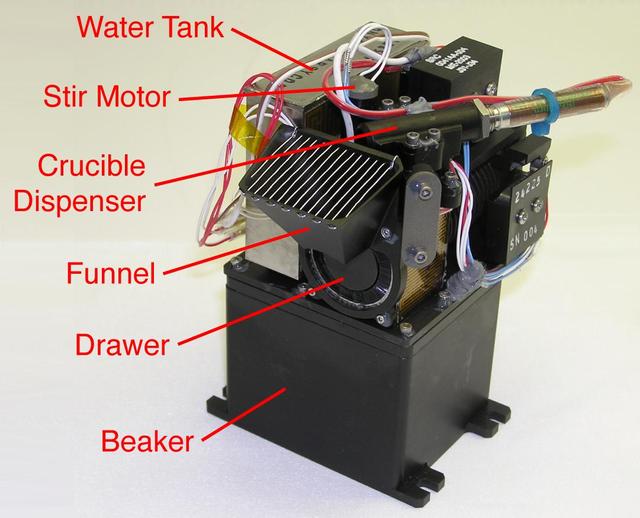
This picture of NASA Phoenix Mars Lander Wet Chemistry Laboratory WCL cell is labeled with components responsible for mixing Martian soil with water from Earth, adding chemicals and measuring the solution chemistry.

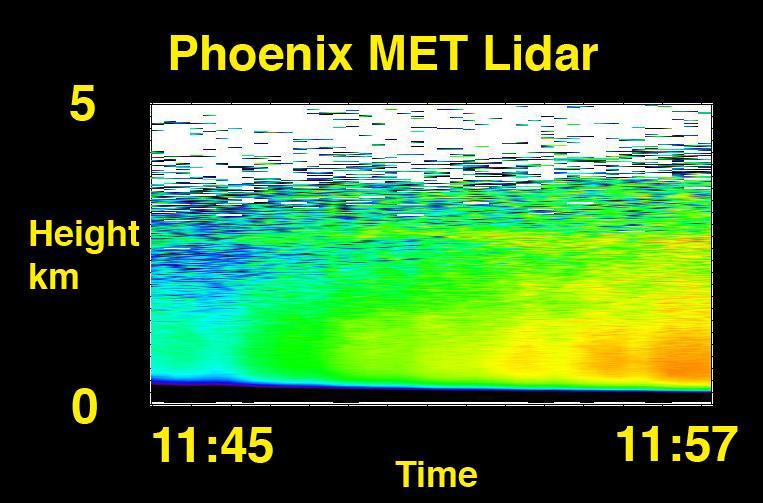
Phoenix Again Carries Soil to Wet Chemistry Lab

Phoenix Carries Soil to Wet Chemistry Lab
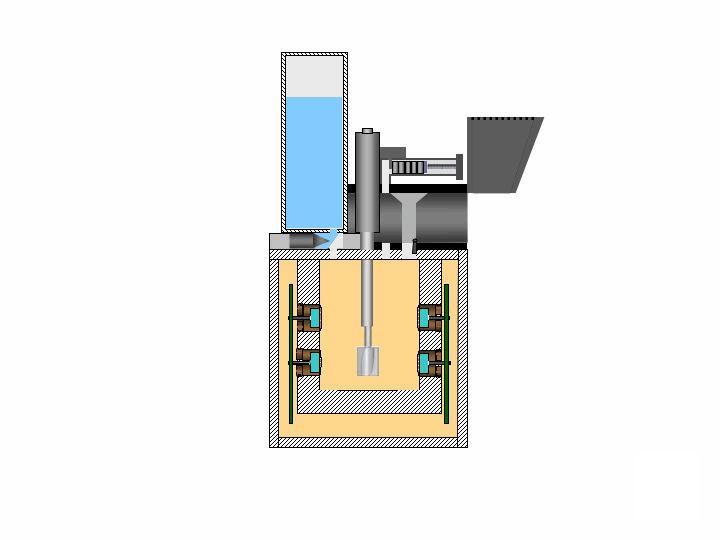
This is an illustration of the analytical procedure of NASA Phoenix Mars Lander Wet Chemistry Lab WCL on board the Microscopy, Electrochemistry, and Conductivity Analyzer MECA instrument.
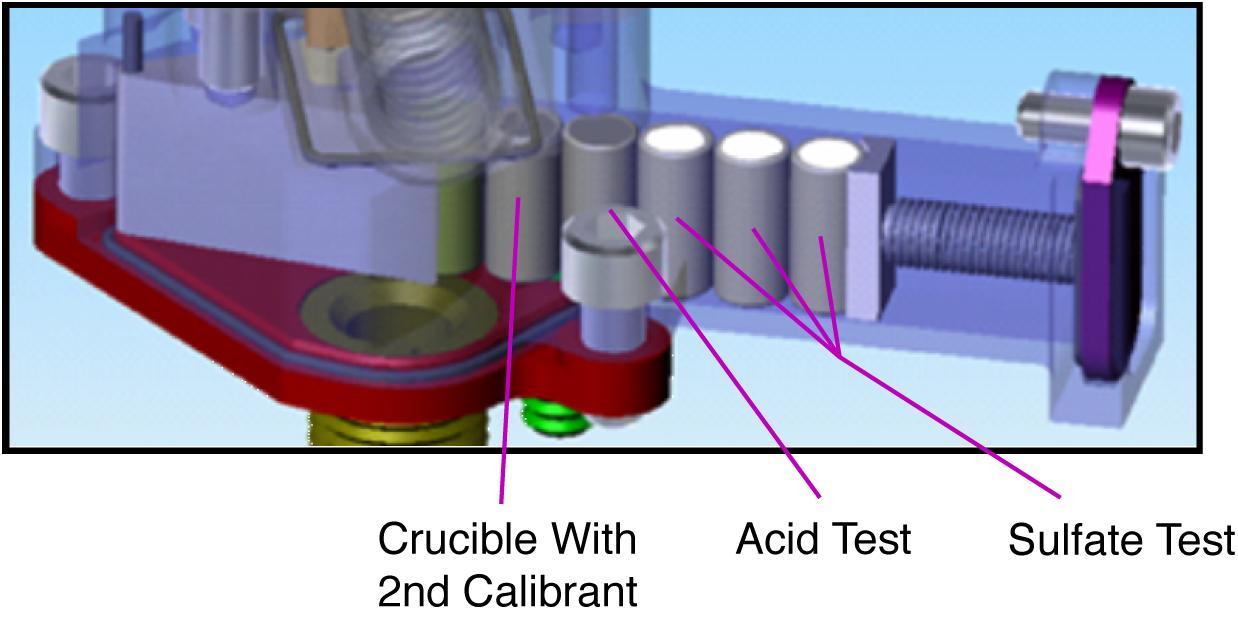
This is an illustration of soil analysis on NASA Phoenix Mars Lander Wet Chemistry Lab WCL on board the Microscopy, Electrochemistry, and Conductivity Analyzer MECA instrument.

This image shows four Wet Chemistry Laboratory units, part of the Microscopy, Electrochemistry, and Conductivity Analyzer MECA instrument on board NASA Phoenix Mars Lander. This image was taken before Phoenix launch on August 4, 2007.

Members of NASA Mars Science Laboratory team carefully steer the hoisted Chemistry and Mineralogy CheMin instrument during its June 15, 2010, installation into the mission Mars rover, Curiosity.

iss062e123434 (4/5/2020) --- A view of the Flow Chemistry Platform for Synthetic Reactions on ISS investigation aboard the International Space Station (ISS). The Flow Chemistry Platform for Synthetic Reactions on ISS investigation studies the effects of microgravity on synthetic chemical reactions as a step toward on-demand production of chemicals and materials in space.
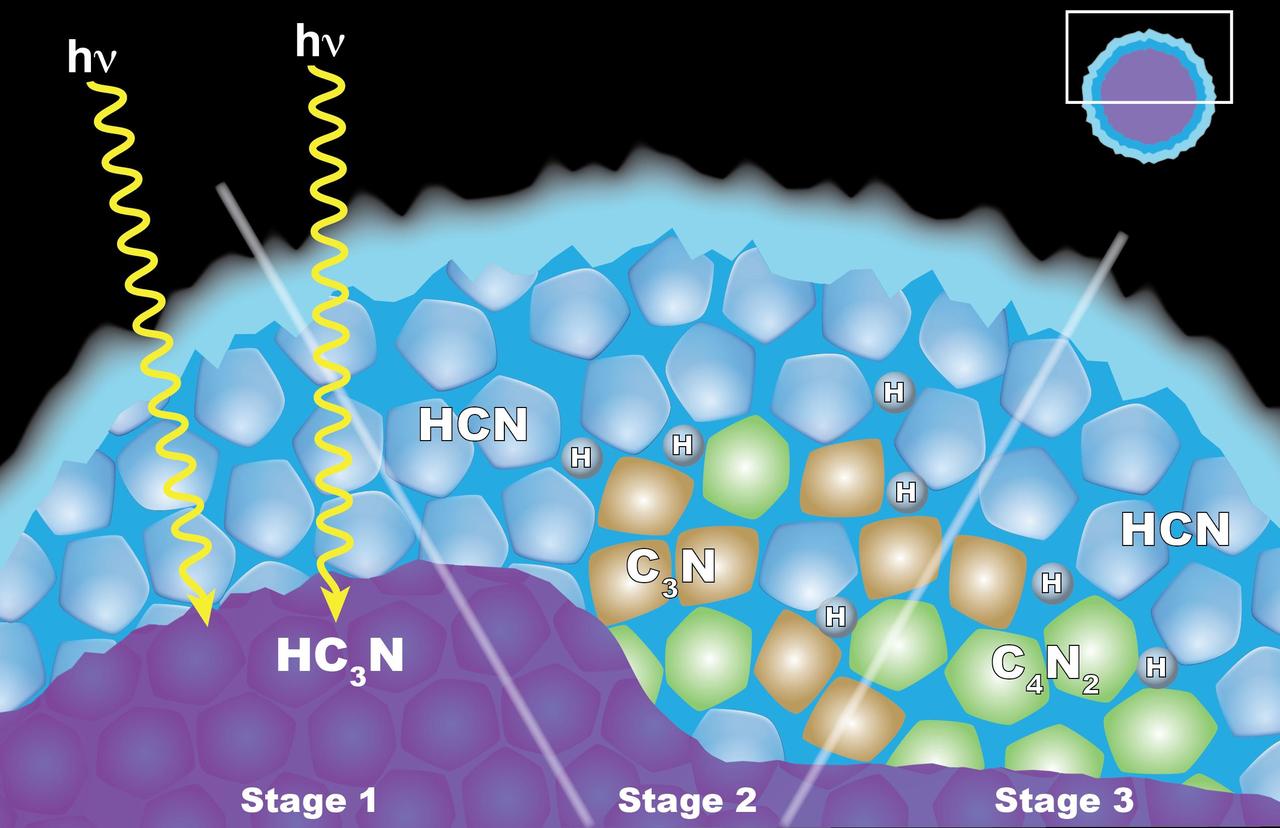
Scientists from NASA's Cassini mission suggested in a 2016 paper that the appearance of a cloud of dicyanoacetylene (C4N2) ice in Titan's stratosphere may be explained by "solid-state" chemistry taking place inside ice particles. The particles have an inner layer of cyanoacetylene (HC3N) ice coated with an outer layer of hydrogen cyanide (HCN) ice. Left: When a photon of light penetrates the outer shell, it can interact with the HC3N, producing C3N and H. Center: The C3N then reacts with HCN to yield C4N2 and H (shown at right). Another reaction that also yields C4N2 ice and H also is possible, but the researchers think it is less likely. http://photojournal.jpl.nasa.gov/catalog/PIA20715

The Aura spacecraft is NASA atmospheric chemistry mission that is monitoring the Earth protective atmosphere.

David Bushman, unmanned aerial vehicle (UAV) mission manager in NASA Dryden's Airborne Science Program, explains the capabilities of the Altus UAV to Charles Hudgins of NASA Langley's Chemistry and Dynamics Branch.
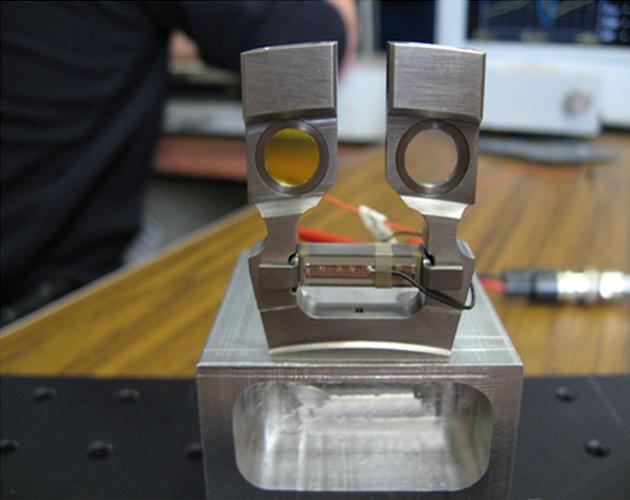
This image shows the cells that hold the soil samples that are vibrated by the Chemistry and Mineralogy CheMin instrument on NASA Curiosity rover.

This image shows the calibration target for the Chemistry and Camera instrument on NASA Curiosity rover before it was installed on the rover and readied for launch.
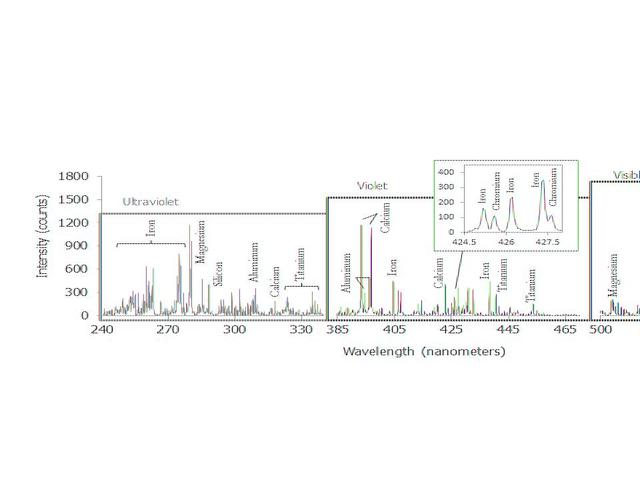
This graph shows a spectrum recorded by the Chemistry and Camera instrument ChemCam in NASA Curiosity Mars rover; it is is typical of Martian volcanic basalt material.
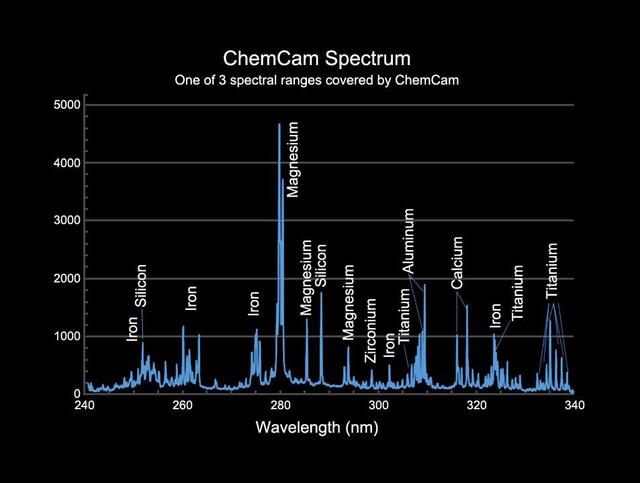
This image provides an example of the type of data collected by the Chemistry and Camera ChemCam instrument on NASA Mars Science Laboratory mission Curiosity rover.

This artist concept depicts the rover Curiosity, of NASA Mars Science Laboratory mission, as it uses its Chemistry and Camera ChemCam instrument to investigate the composition of a rock surface.
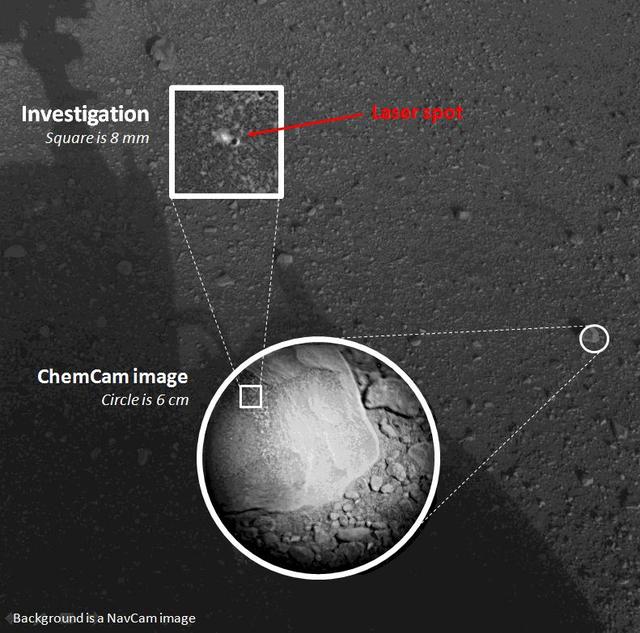
This composite image, with magnified insets, depicts the first laser test by the Chemistry and Camera, or ChemCam, instrument aboard NASA Curiosity Mars rover.
This is an illustration of the analytical procedure of NASA Phoenix Mars Lander Wet Chemistry Lab WCL on board the Microscopy, Electrochemistry, and Conductivity Analyzer MECA instrument.
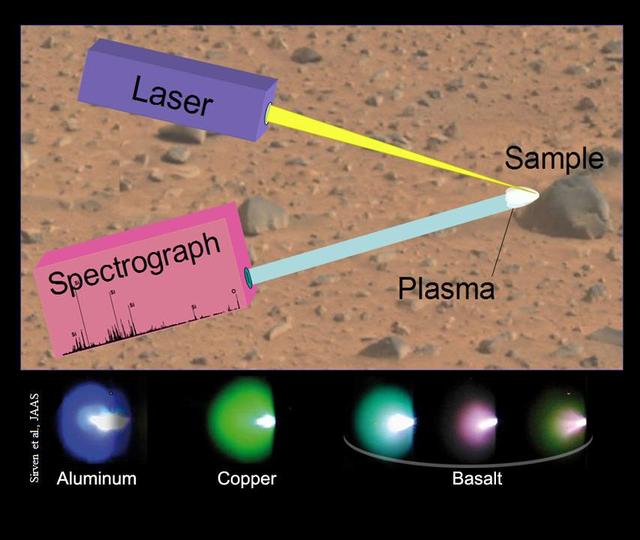
This image illustrates the principals of a technique called laser-induced breakdown spectroscopy, which the Chemistry and Camera ChemCam instrument onboard NASA rover, Curiosity, will use on Mars.

This image displays the type of detail discernable with the telescopic camera of the Chemistry and Camera ChemCam instrument on the Mars Science Laboratory mission Curiosity rover.

These images from the Chemistry and Camera (ChemCam) instrument on NASA's Curiosity Mars rover indicate similarly dark material, but with very different chemistries, in mineral veins at "Garden City." Each of the side-by-side circular images covers an area about 2 inches (5 centimeters) in diameter. The images were taken by ChemCam's Remote Micro-Imager. Researchers used ChemCam's laser, telescope and spectrometers to examine the chemistry of material in these veins. While both of these veins are dark, their chemistries are very different, indicating that they were formed by different fluids. One common aspect of the chemistry in the dark material is an iron content higher than nearby bedrock. Thus the dark appearance may be result of similar iron content. The dark maerial in the vein on the left is enriched in calcium and contains calcium fluorine. The dark material in the vein on the right is enriched in magnesium, but not in calcium or calcium fluorine. Thus, the veins were formed by different fluids that deposited minerals in rock fractures. The Remote Micro-Imager took the image on the left on March 27, 2015, during the 938th Martian day, or sol, of Curiosity's work on Mars. The next day, it took the image on the right. A broader view of the prominent mineral veins at Garden City is at PIA19161. ChemCam is one of 10 instruments in Curiosity's science payload. The U.S. Department of Energy's Los Alamos National Laboratory, in Los Alamos, New Mexico, developed ChemCam in partnership with scientists and engineers funded by the French national space agency (CNES), the University of Toulouse and the French national research agency (CNRS). More information about ChemCam is available at http://www.msl-chemcam.com. http://photojournal.jpl.nasa.gov/catalog/PIA19924

Aaron Beeler, professor of chemistry at Boston University and principal investigator of the Flow Chemistry Platform for Synthetic Reactions on ISS study, addresses NASA Social participants during a What’s on Board science briefing at the agency’s Kennedy Space Center in Florida on March 5, 2020. The briefing provided a closer look at some of the payloads launching on SpaceX’s 20th Commercial Resupply Services (CRS-20) mission to the International Space Station. The company’s Falcon 9 rocket is scheduled to lift off from Cape Canaveral Air Force Station’s Space Launch Complex 40 at 11:50 p.m. EST on March 6, 2020.

Aaron Beeler, professor of chemistry at Boston University and principal investigator of the Flow Chemistry Platform for Synthetic Reactions on ISS study, addresses NASA Social participants during a What’s on Board science briefing at the agency’s Kennedy Space Center in Florida on March 5, 2020. The briefing provided a closer look at some of the payloads launching on SpaceX’s 20th Commercial Resupply Services (CRS-20) mission to the International Space Station. The company’s Falcon 9 rocket is scheduled to lift off from Cape Canaveral Air Force Station’s Space Launch Complex 40 at 11:50 p.m. EST on March 6, 2020.

MSL - Chemistry and Mineralogy Demo by David Blake

MSL - Chemistry and Mineralogy Demo by David Blake

The Sample Analysis at Mars SAM instrument will analyze samples of Martian rock and soil collected by the rover arm to assess carbon chemistry through a search for organic compounds, and to look for clues about planetary change.
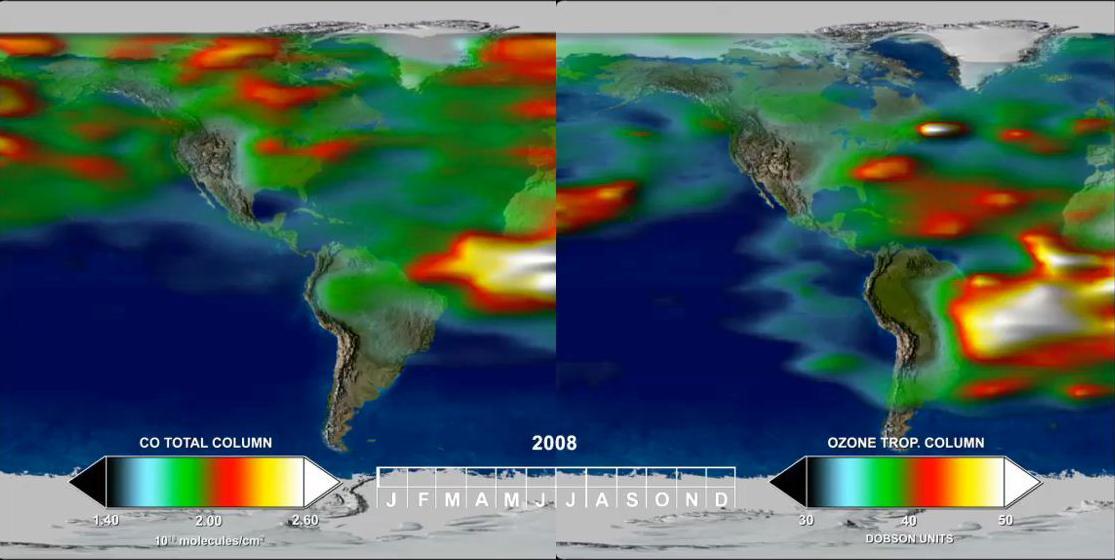
This frame from a time series, from one year of Tropospheric Emission Spectrometer TES measurements, shows how powerful the TES data are for understanding emissions, chemistry, and transport in the troposphere.

The Sample Analysis at Mars SAM instrument for NASA Mars Science Laboratory mission will study chemistry of rocks, soil and air as the mission rover, Curiosity, investigates Gale Crater on Mars.

This mosaic shows the calibration target for the Chemistry and Camera ChemCam instrument on NASA Curiosity rover, as seen by the ChemCam remote micro-imager. The 10 images incorporated in this mosaic were taken on Aug. 15.

NASA Terrestrial Planet Finder will use multiple telescopes working together to take family portraits of stars and their orbiting planets and determine which planets may have the right chemistry to sustain life.

The Chemistry and Camera ChemCam instrument on NASA Mars rover Curiosity used its laser and spectrometers to examine what chemical elements are in a drift of Martian sand during the mission 74th Martian day, or sol Oct. 20, 2012.

A day after NASA Mars rover Curiosity drilled the first sample-collection hole into a rock on Mars, the rover Chemistry and Camera ChemCam instrument shot laser pulses into the fresh rock powder that the drilling generated.
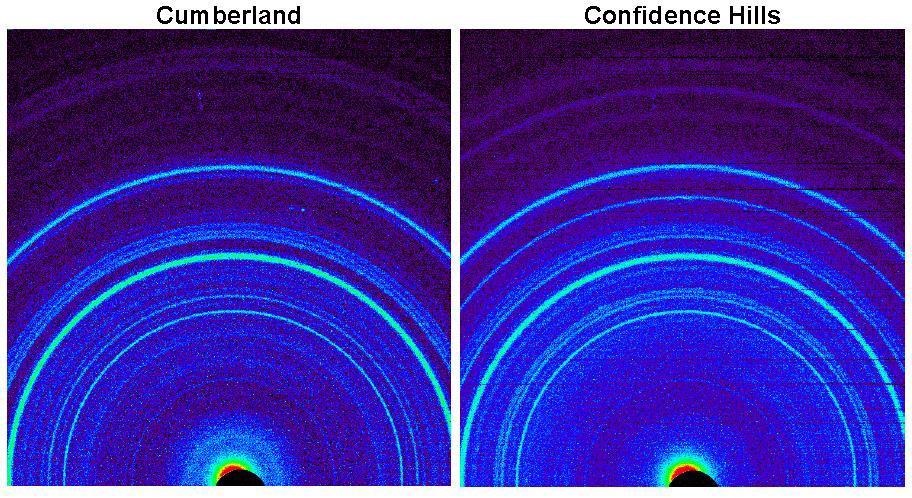
This side-by-side comparison shows the X-ray diffraction patterns of two different samples collected from rocks on Mars by NASA Curiosity rover. The images present data obtained by Curiosity Chemistry and Mineralogy instrument CheMin.

The Chemistry and Camera ChemCam instrument on NASA Mars rover Curiosity used its laser to examine side-by-side points in a target patch of soil, leaving the marks apparent in this before-and-after comparison.

The Chemistry and Camera ChemCam instrument on NASA Mars rover Curiosity was used to check the composition of gray tailings from the hole in rock target Cumberland that the rover drilled on May 19, 2013.

This close-up image shows the first target NASA Curiosity rover aims to zap with its Chemistry and Camera ChemCam instrument. The instrument will analyze that spark with a telescope and identify the chemical elements in the target.

A conventional X-ray diffraction instrument left is the size of a large refrigerator, in contrast to the compact size of the Chemistry and Mineralogy CheMin instrument on NASA Curiosity rover top right.
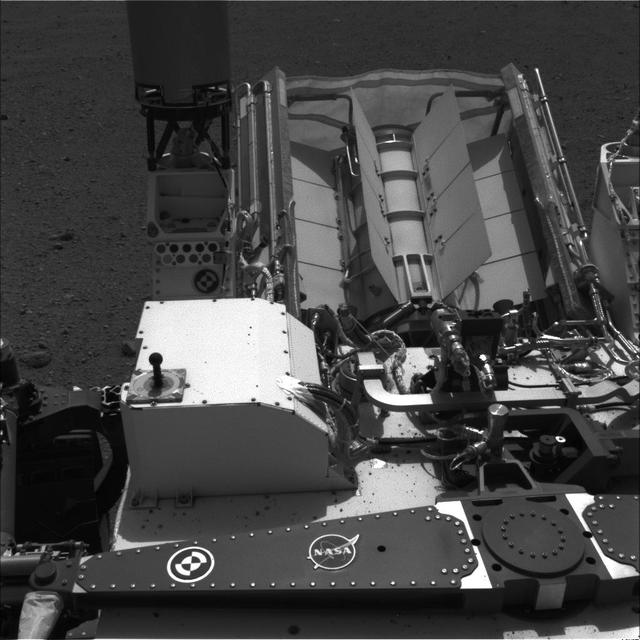
This image shows the calibration target for the Chemistry and Camera ChemCam instrument on NASA Curiosity rover. The calibration target is one square and a group of nine circles that look dark in the black-and-white image.

Researchers prepare for a test of the Chemistry and Camera ChemCam instrument that will fly on NASA Mars Science Laboratory mission; researchers are preparing the instrument mast unit for a laser firing test.
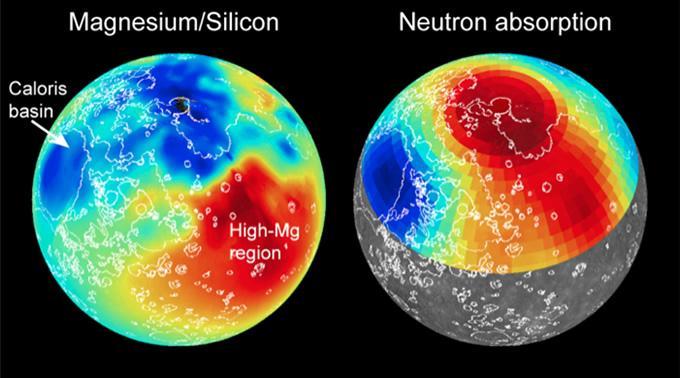
Maps of magnesium/silicon (left) and thermal neutron absorption (right) across Mercury's surface (red indicates high values, blue low) are shown. These maps, together with maps of other elemental abundances, reveal the presence of distinct geochemical terranes. Volcanic smooth plains deposits are outlined in white. Read the mission news story to learn more! http://photojournal.jpl.nasa.gov/catalog/PIA19242
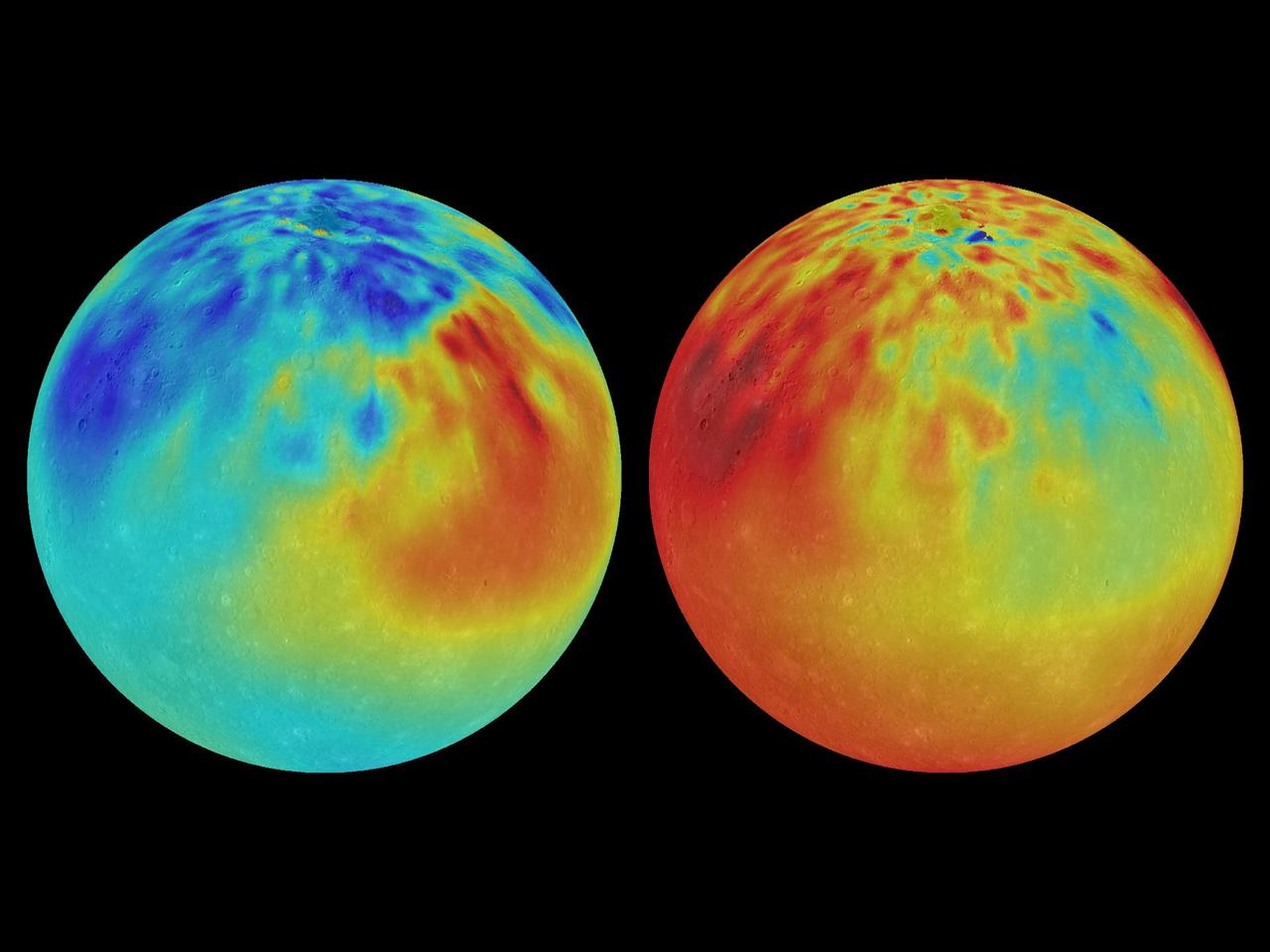
During NASA MESSENGER four-year orbital mission, the spacecraft X-Ray Spectrometer XRS instrument mapped out the chemical composition of Mercury and discovered striking regions of chemical diversity. These maps of magnesium/silicon (left) and aluminium/silicon (right) use red colors to indicate high values and blue colors for low values. In the maps shown here, the Caloris basin can be identified as a region with low Mg/Si and high Ca/Si on the upper left of each map. An extensive region with high Mg/Si is also clearly visible in the maps but is not correlated with any visible impact basin. Instrument: X-Ray Spectrometer (XRS) and Mercury Dual Imaging System (MDIS) Left Image: Map of Mg/Si Right Image: Map of Al/Si http://photojournal.jpl.nasa.gov/catalog/PIA19417
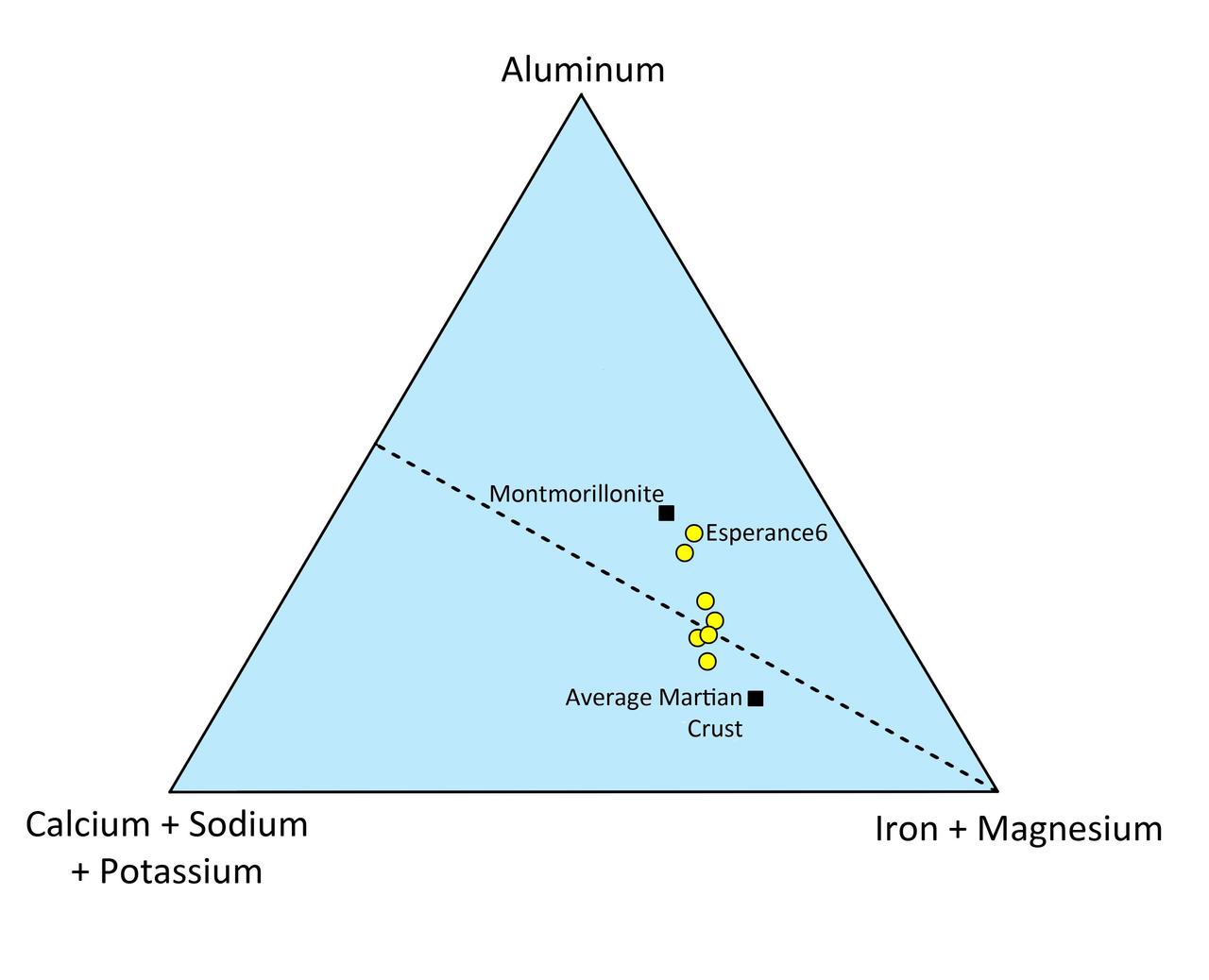
This triangle plot shows the relative concentrations of some of the major chemical elements in the Martian rock Esperance. The compositions of average Martian crust and of montmorillonite, a common clay mineral, are shown.

iss042e006636 (11/18/2014) ---Aboard the International Space Station: this image is a Close-up view of hardware for a chemistry experiment and was taken in the Rassvet Mini-Research Module.

NASA’s ER-2 aircraft performs a check flight for the Dynamics and Chemistry of the Summer Stratosphere, or DCOTSS, 2022 campaign on May 13, 2022.

GATEWAY WORMS! Woodside High School AP Chemistry Class Students and Ames Consultants Dr Beverly Cirten, Mike Eodice, Barbara Navarro (Worms in space)
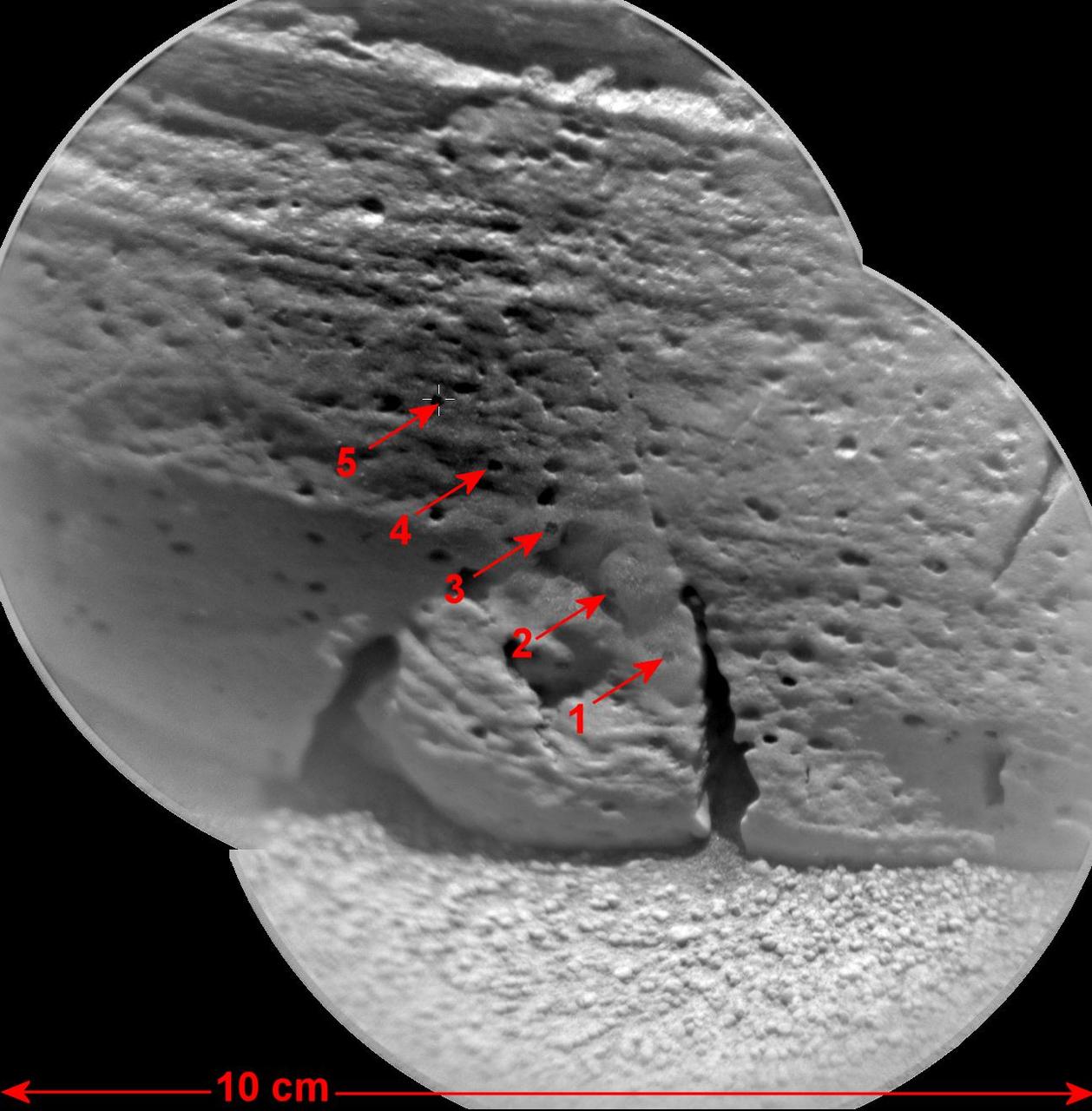
This view of a rock called Rocknest 3 combines two images taken by the Chemistry and Camera ChemCam instrument on the NASA Mars rover Curiosity and indicates five spots where ChemCam had hit the rock with laser pulses to check its composition.

This pair of images taken a few minutes apart show how laser firing by NASA Mars rover Curiosity removes dust from the surface of a rock. The images were taken by the remote micro-imager camera in the laser-firing Chemistry and Camera ChemCam.

Since landing on Mars in August 2012, NASA Curiosity Mars rover has fired the laser on its Chemistry and Camera ChemCam instrument more than 100,000 times at rock and soil targets up to about 23 feet 7 meters away.

In this illustration, NASA's Perseverance rover uses its Planetary Instrument for X-ray Lithochemistry (PIXL) instrument to analyze a rock on the surface of Mars. PIXL uses a focused X-ray beam to analyze the chemistry of features as small as a grain of sand. The tiny but powerful beam causes rocks to fluoresce, or produce a glow. While the glow is invisible to the human eye, it is detectable by the instrument and varies according to the rock's elemental chemistry. PIXL scan the beam across the surface of the rock to produce a postage-stamp-sized map of the rock's chemistry at the end of an overnight scan. PIXL also has an optical fiducial system (OFS) that includes white "flood lights" — seen on the rock in this artist's concept — that is used together with a pattern of red lasers to illuminate the rock while its camera captures images of the mapped area. The pictures are used for multiple purposes: PIXL analyzes them on board to work out where the target is — in 3D — and move itself into the right position for science collection and the safety of the instrument. The pictures are also downlinked to Earth so scientists can see exactly where each measurement was taken. This allows scientists to tie chemistry accurately to rock texture, which helps them to determine how these features formed and whether they were biological in nature. https://photojournal.jpl.nasa.gov/catalog/PIA23719

NASA’s Armstrong Flight Research Center ER-2 #809 high-altitude aircraft prepped for Dynamics and Chemistry of the Summer Stratosphere (DCOTSS) science flights in Palmdale, CA.

NASA's Armstrong Flight Research Center ER-2 pilot Gary Toroni and engineering technician Raul "Corky" Cortes preparing for Dynamics and Chemistry of the Summer Stratosphere (DCOTSS) science flights in Palmdale, CA on June 17, 2021.

NASA's Armstrong Flight Research Center operates ER-2 #809 high-altitude aircraft for Dynamics and Chemistry of the Summer Stratosphere (DCOTSS) science flights on June 17, 2021.

NASA's Armstrong Flight Research Center ER-2 #809 high-altitude aircraft prepared for Dynamics and Chemistry of the Summer Stratosphere (DCOTSS) science flights in Palmdale, CA on June 17, 2021.

NASA’s Armstrong Flight Research Center ER-2 #809 high-altitude aircraft maintained by avionics technician Gregory Bantalin for Dynamics and Chemistry of the Summer Stratosphere (DCOTSS) science flights.

NASA's Armstrong Flight Research Center ER-2 #809 high-altitude aircraft prepped for Dynamics and Chemistry of the Summer Stratosphere (DCOTSS) science flights in Palmdale, CA.

NASA's Armstrong Flight Research Center ER-2 #809 high-altitude aircraft prepped for Dynamics and Chemistry of the Summer Stratosphere (DCOTSS) science flights in Palmdale, CA.
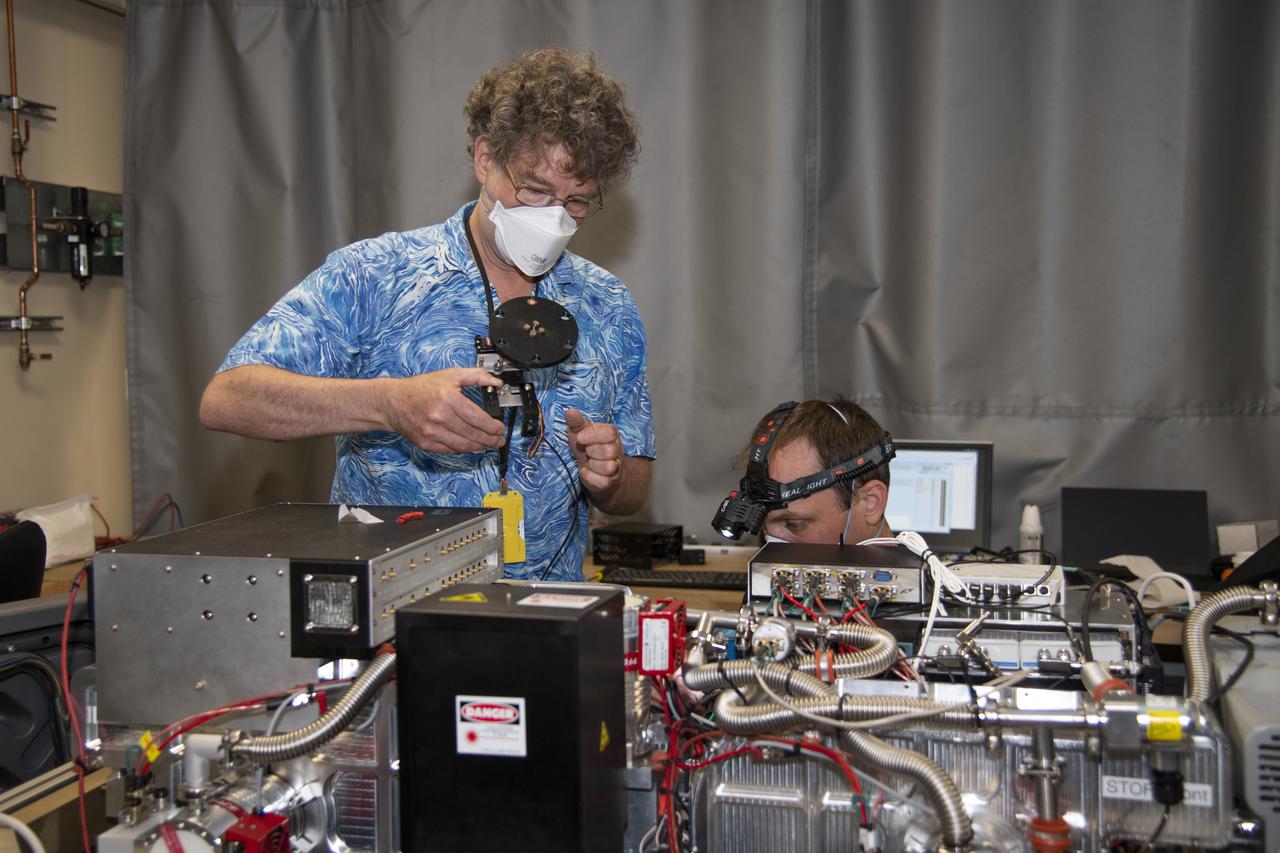
Particle Analysis by Laser Mass Spectrometry or PALMS instrument maintained by Dave Thomson and Justin Jacquot of Purdue University as part of NASA's Dynamics and Chemistry of the Summer Stratosphere (DCOTSS) mission

NASA's Armstrong Flight Research Center ER-2 #809 high-altitude aircraft prepared for Dynamics and Chemistry of the Summer Stratosphere (DCOTSS) science flights in Palmdale, CA on June 17, 2021

The Harvard Halogen Instrument (HAL) is prepared for integration on NASA's ER-2 by electrical engineer Marco Rivero and mechanical engineer Michael Greenberg for the Dynamics and Chemistry of the Summer Stratosphere, or DCOTSS, 2022 campaign.

NASA’s Armstrong Flight Research Center ER-2 #809 high-altitude aircraft taking off for Dynamics and Chemistry of the Summer Stratosphere (DCOTSS) science flights in Palmdale, CA on June 17, 2021.

NASA's Armstrong Flight Research Center ER-2 #809 high-altitude aircraft prepped for Dynamics and Chemistry of the Summer Stratosphere (DCOTSS) science flights in Palmdale, CA.

NASA’s Armstrong Flight Research Center operates ER-2 #809 high-altitude aircraft for Dynamics and Chemistry of the Summer Stratosphere (DCOTSS) science flights on June 17, 2021.

The targeted landing site for NASA Phoenix Mars Lander is at about 68 degrees north latitude, 233 degrees east longitude in the Martian arctic. The Phoenix lander, which landed May 25, 2008 ceased its operations about six months later.

This STS-48 onboard photo is of the Upper Atmosphere Research Satellite (UARS) in the grasp of the RMS (Remote Manipulator System) during deployment, September 1991. UARS gathers data related to the chemistry, dynamics, and energy of the ozone layer. UARS data is used to study energy input, stratospheric photo chemistry, and upper atmospheric circulation. UARS helps us understand and predict how the nitrogen and chlorine cycles, and the nitrous oxides and halo carbons which maintain them, relate to the ozone balance. It also observes diurnal variations in short-lived stratospheric chemical species important to ozone destruction. Data from UARS enables scientists to study ozone depletion in the upper atmosphere.

This STS-48 onboard photo is of the Upper Atmosphere Research Satellite (UARS) in the grasp of the RMS (Remote Manipulator System) during deployment, September 1991. UARS gathers data related to the chemistry, dynamics, and energy of the ozone layer. UARS data is used to study energy input, stratospheric photo chemistry, and upper atmospheric circulation. UARS helps us understand and predict how the nitrogen and chlorine cycles, and the nitrous oxides and halo carbons which maintain them, relate to the ozone balance. It also observes diurnal variations in short-lived stratospheric chemical species important to ozone destruction. Data from UARS enables scientists to study ozone depletion in the upper atmosphere.
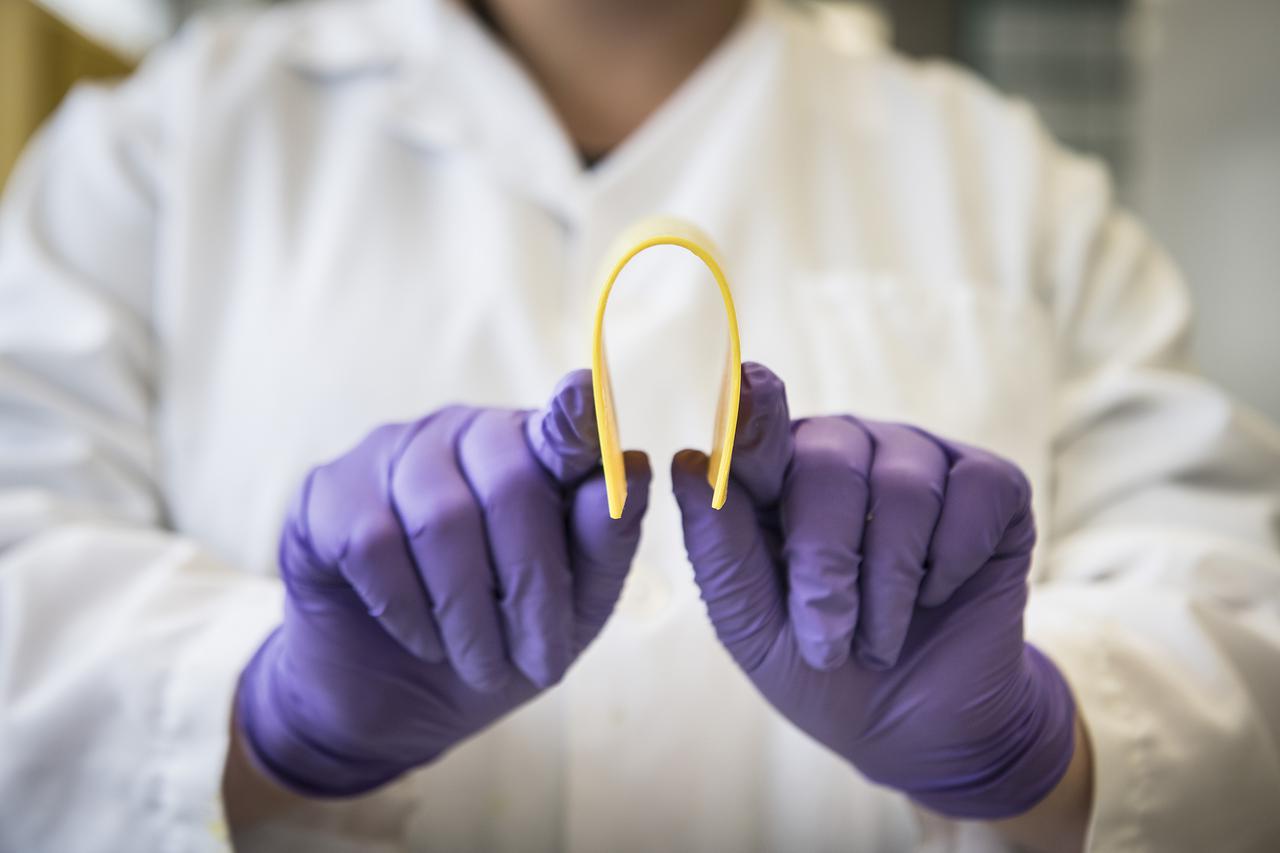
BAPx Flexible Aerogels

These images and overlay bar charts from the Chemistry and Camera (ChemCam) instrument on NASA's Curiosity Mars rover indicate where some high-potassium material is localized within mineral veins at "Garden City." The two images are from ChemCam's Remote Micro-Imager. Each covers an area just over an inch wide (scale bars are in millimeters) in veins at the Garden City site on lower Mount Sharp. The overlay charts show comparisons of potassium (blue) and iron (red) in the mineral veins' compositions determined by reading the spectra of light induced by zapping points in each area with ChemCam's laser. Mineral veins such as these form where fluids move through fractured rocks, depositing minerals in the fractures and affecting chemistry of the surrounding rock. The thin layer of dark fracture-filling material in the image on the right contains much more potassium than the other local material on the left, indicating either different fluid compositions or local variations in the rock. The image on the left was taken on April 4, 2015, during the 946th Martian day, or sol, of Curiosity's work on Mars. The image on the right was taken on Sol 936, on March 25, 2015. A broader view of the prominent mineral veins at Garden City is at PIA19161. http://photojournal.jpl.nasa.gov/catalog/PIA19923

NASA’s Student Airborne Research Program invites Dr. Ann Marie Carlton, Professor of Chemistry at the University of California, Irvine and White House Office of Science and Technology Policy fellow, to fly aboard the DC-8 to measure air quality on June 23, 2022.

NASA’s ER-2 No. 806 returns to flying high-altitude on April 7, 2022, after three years of heavy maintenance. NASA Armstrong operates two ER-2 aircraft to collect information about Earth resources, celestial observations, atmospheric chemistry and dynamics, and oceanic processes.

NASA’s ER-2 No. 806 returns to flying high-altitude on April 7, 2022, after three years of heavy maintenance. NASA Armstrong operates two ER-2 aircraft to collect information about Earth resources, celestial observations, atmospheric chemistry and dynamics, and oceanic processes.

NASA’s Student Airborne Research Program invites Dr. Ann Marie Carlton, Professor of Chemistry at the University of California, Irvine and White House Office of Science and Technology Policy fellow, to fly aboard the DC-8 to measure air quality on June 23, 2022.

NASA's ER-2 high altitude aircraft takes off from Armstrong Flight Research Center Building 703 in Palmdale, California to perform a check flight for the the Dynamics and Chemistry of the Summer Stratosphere, or DCOTSS, 2022 campaign on May 13, 2022.

iss056e101065 (Aug. 1, 2018) --- Expedition 56 Commander Drew Feustel displays TangoLab hardware as Flight Engineer Alexander Gerst looks on. The TangoLab facilities house experimental modules called CubeLabs that enable research into plant biology, microbiology, cell culture, tissue culture, and flow chemistry.

NASA’s Student Airborne Research Program invites Dr. Ann Marie Carlton, Professor of Chemistry at the University of California, Irvine and White House Office of Science and Technology Policy fellow, to fly aboard the DC-8 to measure air quality on June 23, 2022.

NASA’s Student Airborne Research Program invites Dr. Ann Marie Carlton, Professor of Chemistry at the University of California, Irvine and White House Office of Science and Technology Policy fellow, to fly aboard the DC-8 to measure air quality on June 23, 2022.

NASA's ER-2 No. 806 returns to flying high-altitude on April 7, 2022, after three years of heavy maintenance. NASA Armstrong operates two ER-2 aircraft to collect information about Earth resources, celestial observations, atmospheric chemistry and dynamics, and oceanic processes.

NASA’s Student Airborne Research Program invites Dr. Ann Marie Carlton, Professor of Chemistry at the University of California, Irvine and White House Office of Science and Technology Policy fellow, to fly aboard the DC-8 to measure air quality on June 23, 2022.

NASA’s Student Airborne Research Program invites Dr. Ann Marie Carlton, Professor of Chemistry at the University of California, Irvine and White House Office of Science and Technology Policy fellow, to fly aboard the DC-8 to measure air quality on June 23, 2022.

KENNEDY SPACE CENTER, FLA. -- New methods of environmental cleanup are explained during a presentation to government and business representatives, scientists and engineers at Launch Complex 34-A, Cape Canaveral Spaceport. At left is Laura Filipek, a University of Central Florida graduate chemistry student involved in the science.
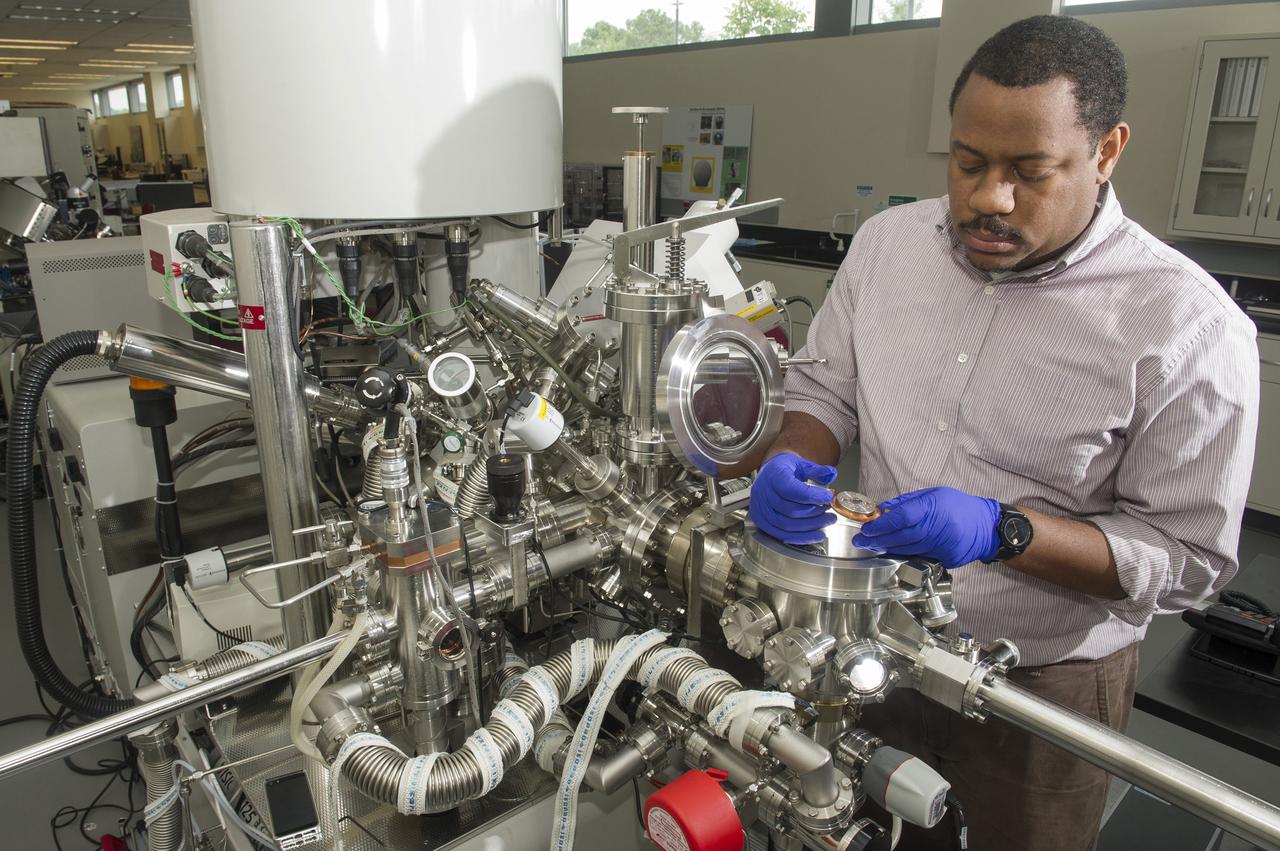
ARTHUR BROWN (AST, AEROSPACE METALLIC MATERIALS) LOADS A CERAMIC COATED SILICON WAFER INTO A KRATOS (ELECTRON SPECTROSCOPY FOR CHEMICAL ANALYSIS) TO PERFORM X-RAY PHOTOELECTRON SPECTROSCOPY (XPS). XPS IS A TECHNIQUE THAT ANALYZES THE SURFACE CHEMISTRY OF A SAMPLE BY IRRADIATING IT WITH X-RAYS AND MEASURING THE NUMBER AND KINETIC ENERGY OF ELECTRON THAT ESCAPE.

NASA Student Airborne Research Program Manager, Dr. Brenna Biggs and Professor of Chemistry at the University of California, Irvine and White House Office of Science and Technology Policy fellow, Dr. Ann Marie Carlton pose in front of the DC-8 on June 23, 2022.

NASA’s Student Airborne Research Program invites Dr. Ann Marie Carlton, Professor of Chemistry at the University of California, Irvine and White House Office of Science and Technology Policy fellow, to fly aboard the DC-8 to measure air quality on June 23, 2022.

NASA Student Airborne Research Program Manager, Dr. Brenna Biggs and Professor of Chemistry at the University of California, Irvine and White House Office of Science and Technology Policy fellow, Dr. Ann Marie Carlton pose in front of the DC-8 on June 23, 2022.

NASA’s Student Airborne Research Program invites Dr. Ann Marie Carlton, Professor of Chemistry at the University of California, Irvine and White House Office of Science and Technology Policy fellow, to fly aboard the DC-8 to measure air quality on June 23, 2022.

NASA’s Student Airborne Research Program invites Dr. Ann Marie Carlton, Professor of Chemistry at the University of California, Irvine and White House Office of Science and Technology Policy fellow, to fly aboard the DC-8 to measure air quality on June 23, 2022.

ISS006-E-27226 (17 February 2003) --- Astronaut Kenneth D. Bowersox, Expedition Six mission commander, uses the water microbiology kit (WMK) to collect water samples for in-flight chemistry/microbiology analysis in the Destiny laboratory on the International Space Station (ISS).

NASA’s Student Airborne Research Program invites Dr. Ann Marie Carlton, Professor of Chemistry at the University of California, Irvine and White House Office of Science and Technology Policy fellow, to fly aboard the DC-8 to measure air quality on June 23, 2022.

One part of the MECA instrument for NASA Phoenix Mars Lander is a pair of telescopes with a special wheel on the right in this photograph for presenting samples to be inspected with the microscopes.

Deborah Efua Adu Essumang, system lead scientist, conducts testing of the Volatile Monitoring Oxygen Measurement Subsystem (VMOMS) for Molten Regolith Electrolysis (MRE) inside a laboratory in the Neil A. Armstrong Operations and Checkout Building at NASA’s Kennedy Space Center in Florida on April 19, 2024. The high-temperature electrolytic process aims to extract oxygen from simulated lunar regolith which will be critical to the agency’s Artemis campaign. Oxygen extracted from the Moon can be utilized for propellent to NASA’s lunar landers, breathable oxygen for astronauts, and a variety of other industrial and scientific applications for NASA’s future missions to the Moon.

Dr. Joel Olson, subject matter expert, conducts testing of the Volatile Monitoring Volatile Monitoring Oxygen Measurement Subsystem (VMOMS) for Molten Regolith Electrolysis (MRE) inside a laboratory in the Neil A. Armstrong Operations and Checkout Building at NASA’s Kennedy Space Center in Florida on April 19, 2024. The high-temperature electrolytic process aims to extract oxygen from simulated lunar regolith which will be critical to the agency’s Artemis campaign. Oxygen extracted from the Moon can be utilized for propellent to NASA’s lunar landers, breathable oxygen for astronauts, and a variety of other industrial and scientific applications for NASA’s future missions to the Moon.