
The bumpy exterior of the turnip yellow mosaic virus (TYMV) protein coat, or capsid, was defined in detail by Dr. Alexander McPherson of the University of California, Irvin using proteins crystallized in space for analysis on Earth. TYMV is an icosahedral virus constructed from 180 copies of the same protein arranged into 12 clusters of five proteins (pentamers), and 20 clusters of six proteins (hexamers). The final TYMV structure led to the unexpected hypothesis that the virus releases its RNA by essentially chemical-mechanical means. Most viruses have fairly flat coats, but in TYNV, the fold in each protein, called the jellyroll, is clustered at the points where the protein pentamers and hexamers join. The jellyrolls are almost standing on end, producing a bumpy surface with knobs at all of the pentamers and hexamers. At the inside surface of the pentamers is a void that is not present at the hexamers. The coating had been seen in early stuties of TYMV, but McPherson's atomic structure shows much more detail. The inside surface is strikingly, and unexpectedly, different than the outside. While the pentamers contain a central void on the inside, the hexameric units contain peptides linked to each other, forming a ring or, more accurately, rings to fill the void. Credit: Dr. Alexander McPherson, University of California, Irvine
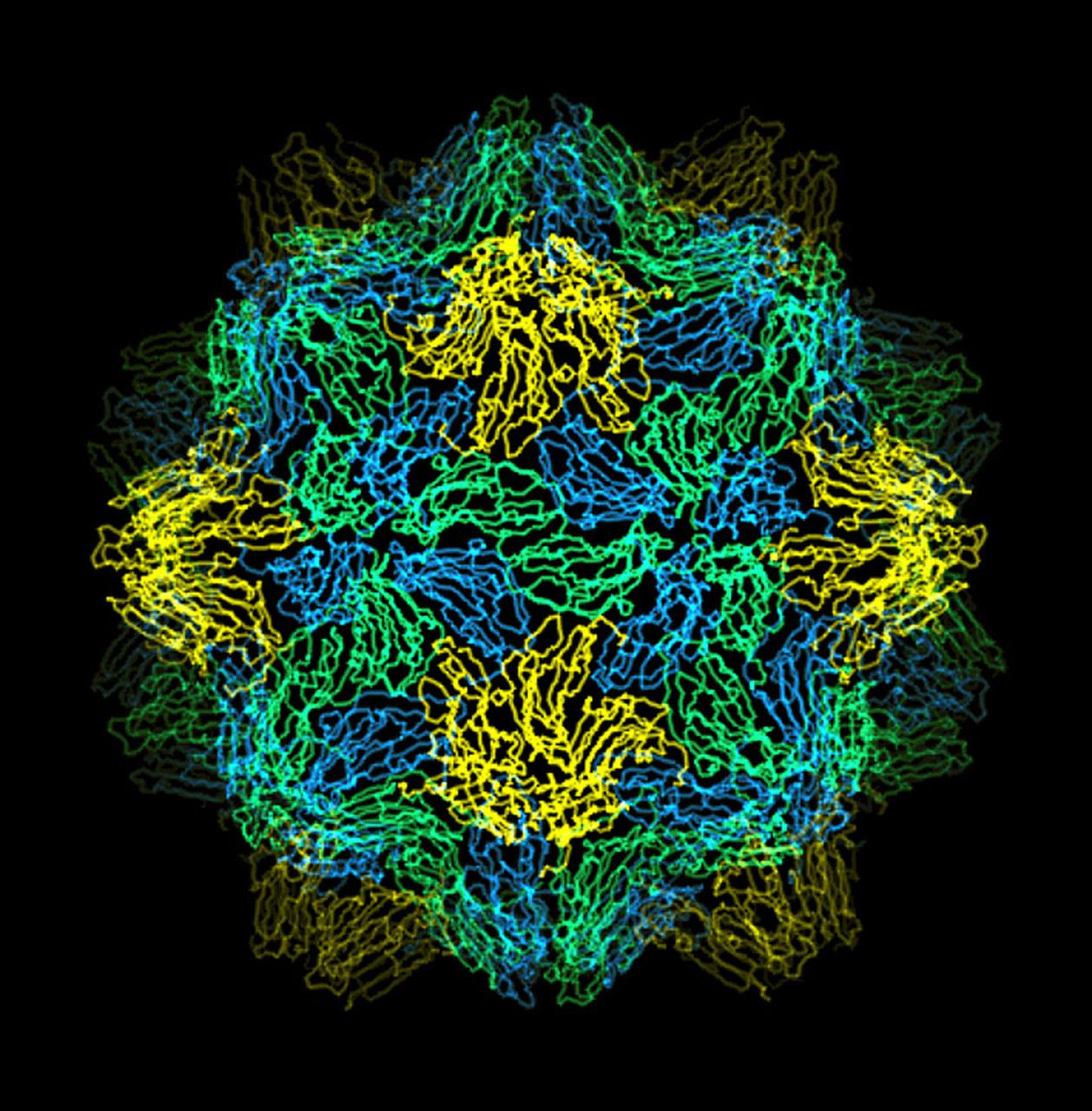
The bumpy exterior of the turnip yellow mosaic virus (TYMV) protein coat, or capsid, was defined in detail by Dr. Alexander McPherson of the University of California, Irvin using protein crystallized in space for analysis on Earth. TYMV is an icosahedral virus constructed from 180 copies of the same protein arranged into 12 clusters of five proteins (pentamers), and 20 clusters of six proteins (hexamers). The final TYMV structure led to the enexpected hypothesis that the virus release its RNA by essentially chemical-mechanical means. Most viruses have farly flat coats, but in TYMV, the fold in each protein, called the jellyroll, is clustered at the points where the protein pentamers and hexamers join. The jellyrolls are almost standing on end, producing a bumpy surface with knobs at all of the pentamers and hexamers. At the inside surface of the pentamers is a void that is not present at the hexamers. The coating had been seen in early studies of TYMV, but McPhereson's atomic structure shows much more detail. The inside surface is strikingly, and unexpectedly, different than the outside. While the pentamers contain a central viod on the inside, the hexameric units contain peptides liked to each other, forming a ring or, more accurately, rings to fill the voild. Credit: Dr. Alexander McPherson, University of California, Irvine.

The Honorable Edward R. McPherson talks during the NASA Advisory Council meeting held at the Rayburn House Office Building, Tuesday, Nov. 29, 2005, In Washington. Photo Credit: (NASA/Bill Ingalls)
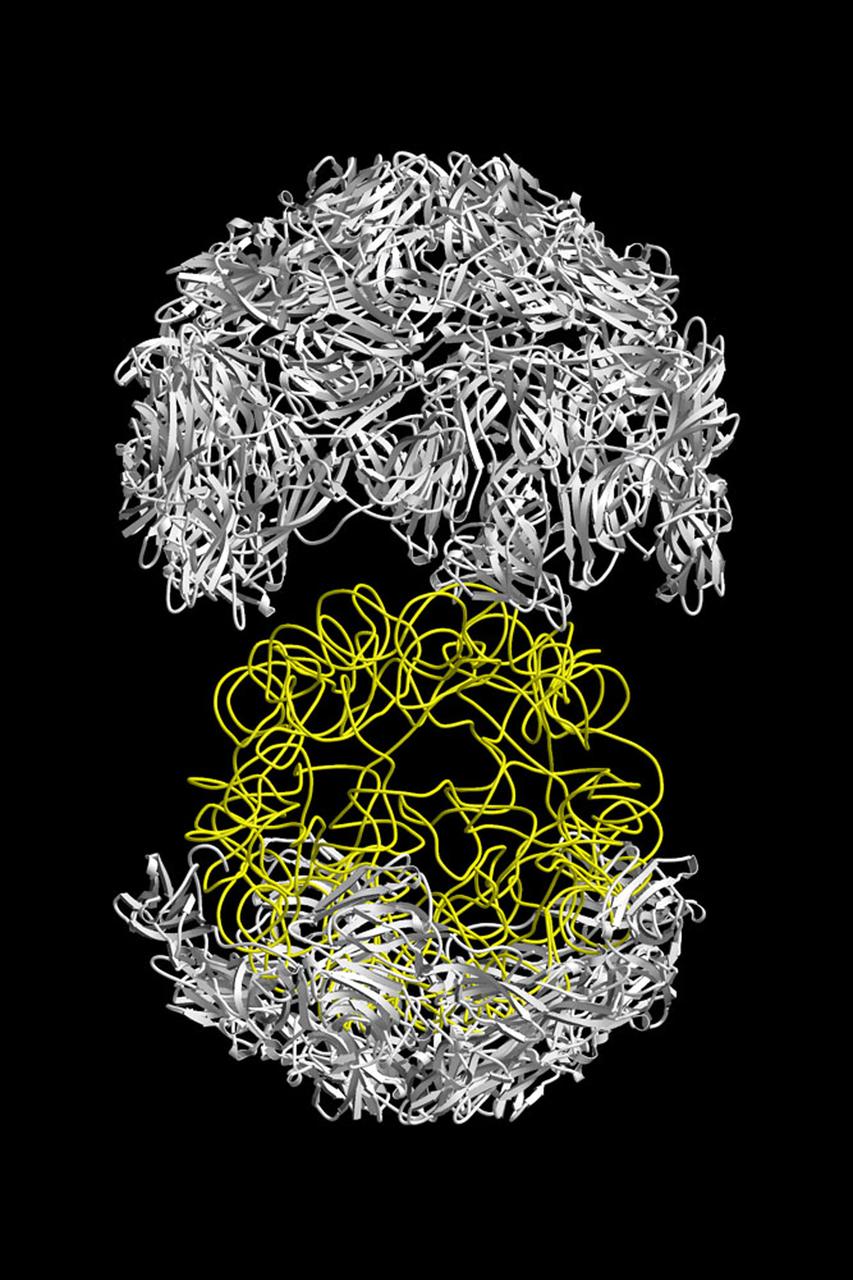
The structure of the Satellite Tobacco Mosaic Viurus (STMV)--one of the smallest viruses known--has been successfully reduced using STMV crystals grown aboard the Space Shuttle in 1992 and 1994. The STMV crystals were up to 30 times the volume of any seen in the laboratory. At the time they gave the best resolution data ever obtained on any virus crystal. STMV is a small icosahedral plant virus, consisting of a protein shell made up of 60 identical protein subunits of molecular weight 17,500. Particularly noteworthy is the fact that, in contrast to the crystals grown on Earth, the crystals grown under microgravity conditions were visually perfect, with no striations or clumping of crystals. Furthermore, the x-ray diffraction data obtained from the space-grown crystals was of a much higher quality than the best data available at that time from ground-based crystals. This stylized ribbon model shows the protein coat in white and the nucleic acid in yellow. STMV is used because it is a simple protein to work with; studies are unrelated to tobacco. Credit: Dr. Alex McPherson, University of California at Irvin.
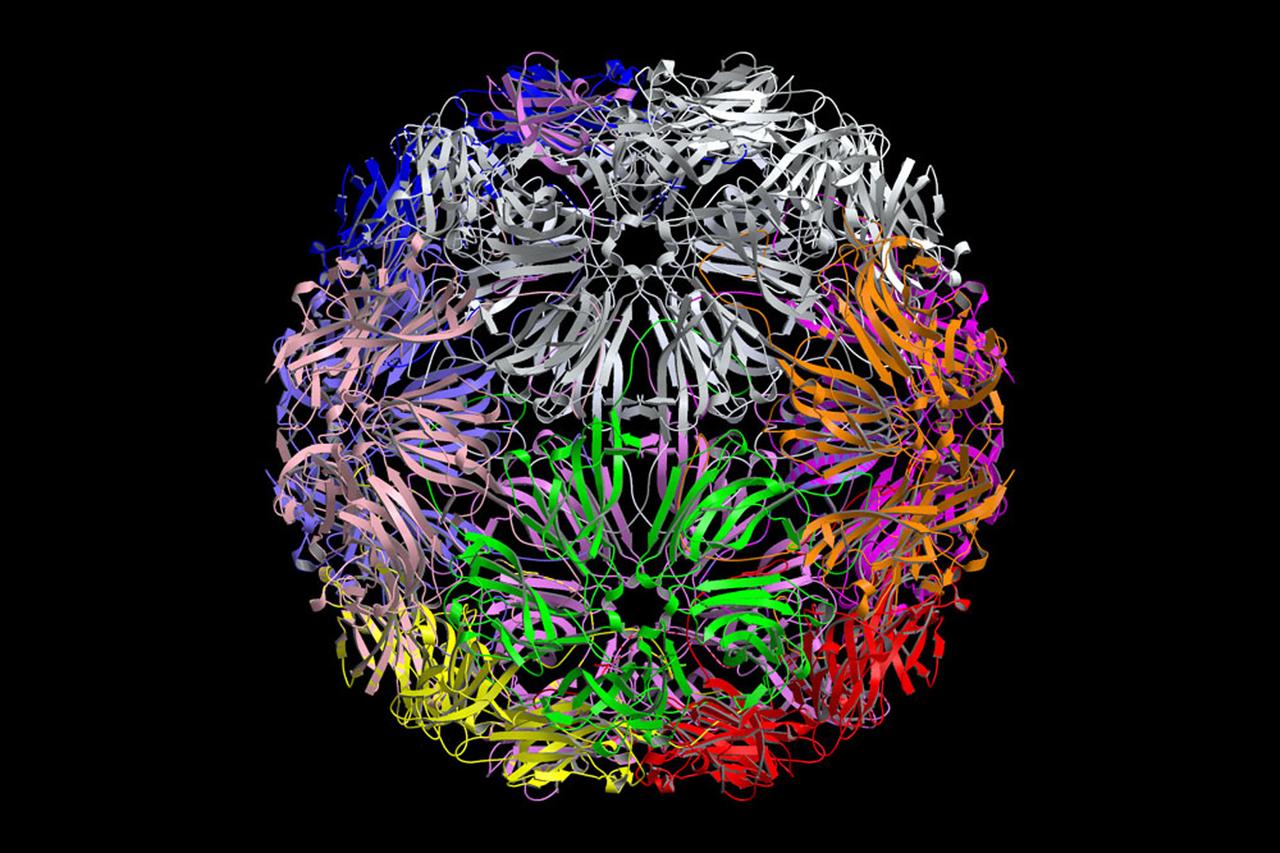
The structure of the Satellite Tobacco Mosaic Virus (STMV)--one of the smallest viruses known--has been successfully deduced using STMV crystals grown aboard the Space Shuttle in 1992 and 1994. The STMV crystals were up to 30 times the volume of any seen in the laboratory. At the same time they gave the best resolution data ever obtained on any virus crystal. STMV is a small icosahedral plant virus, consisting of a protein shell made up of 60 identical protein subunits of molecular weight 17,500. Particularly noteworthy is the fact that, in contrast to the crystal grown on Earth, the crystals grown under microgravity conditions were viusally perfect, with no striations or clumping of crystals. Furthermore, the X-ray diffraction data obtained from the space-grown crystals was of a much higher quality than the best data available at that time from ground-based crystals. This computer model shows the external coating or capsid. STMV is used because it is a simple protein to work with; studies are unrelated to tobacco. Credit: Dr. Alex McPherson, Univeristy of California at Irvin.
Front view of Observable Protein Crystal Growth Apparatus (OPCGA) experiment residing in a Thermal Enclosure System (TES). Principal Investigator is Alexander McPherson. First flight plarned for ISS.

Joel Kearns viewing a laboratory demonstration of the Observable Protein Crystal Growth Apparatus (OPCGA) experiment module. Principal Investigator is Alexander McPherson. First flight plarned for ISS.
![Atomic force microscopy uses laser technology to reveal a defect, a double-screw dislocation, on the surface of this crystal of canavalin, a major source of dietary protein for humans and domestic animals. When a crystal grows, attachment kinetics and transport kinetics are competing for control of the molecules. As a molecule gets close to the crystal surface, it has to attach properly for the crystal to be usable. NASA has funded investigators to look at those attachment kinetics from a theoretical standpoint and an experimental standpoint. Dr. Alex McPherson of the University of California, Irvine, is one of those investigators. He uses X-ray diffraction and atomic force microscopy in his laboratory to answer some of the many questions about how protein crystals grow. Atomic force microscopy provides a means of looking at how individual molecules are added to the surface of growing protein crystals. This helps McPherson understand the kinetics of protein crystal growth. McPherson asks, How fast do crystals grow? What are the forces involved? Investigators funded by NASA have clearly shown that such factors as the level of supersaturation and the rate of growth all affect the habit [characteristic arrangement of facets] of the crystal and the defects that occur in the crystal.](https://images-assets.nasa.gov/image/0101744/0101744~small.jpg)
Atomic force microscopy uses laser technology to reveal a defect, a double-screw dislocation, on the surface of this crystal of canavalin, a major source of dietary protein for humans and domestic animals. When a crystal grows, attachment kinetics and transport kinetics are competing for control of the molecules. As a molecule gets close to the crystal surface, it has to attach properly for the crystal to be usable. NASA has funded investigators to look at those attachment kinetics from a theoretical standpoint and an experimental standpoint. Dr. Alex McPherson of the University of California, Irvine, is one of those investigators. He uses X-ray diffraction and atomic force microscopy in his laboratory to answer some of the many questions about how protein crystals grow. Atomic force microscopy provides a means of looking at how individual molecules are added to the surface of growing protein crystals. This helps McPherson understand the kinetics of protein crystal growth. McPherson asks, How fast do crystals grow? What are the forces involved? Investigators funded by NASA have clearly shown that such factors as the level of supersaturation and the rate of growth all affect the habit [characteristic arrangement of facets] of the crystal and the defects that occur in the crystal.
The major storage protein of leguminous plants and a major source of dietary protein for humans and domestic animals. It is studied in efforts to enhance nutritional value of proteins through protein engineerings. It is isolated from Jack Bean because of it's potential as a nutritional substance. Principal Investigator was Alexander McPherson.

Astronaut Michael Clifford places a liquid nitrogen Dewar containing frozen protein solutions aboard Russia's space station Mir during a visit by the Space Shuttle (STS-76). The protein samples were flash-frozen on Earth and will be allowed to thaw and crystallize in the microgravity environment on Mir Space Station. A later crew will return the Dewar to Earth for sample analysis. Dr. Alexander McPherson of the University of California at Riverside is the principal investigator. Photo credit: NASA/Johnson Space Center.
(PCG) Protein Crystal Growth Canavalin. The major storage protein of leguminous plants and a major source of dietary protein for humans and domestic animals. It is studied in efforts to enhance nutritional value of proteins through protein engineerings. It is isolated from Jack Bean because of it's potential as a nutritional substance. Principal Investigator on STS-26 was Alex McPherson.

Astronaut Tom Akers places a liquid nitrogen Dewar containing frozen protein solutions aboard Russia's space Station Mir during a visit by the Space Shuttle (STS-79). The protein samples were flash-frozen on Earth and will be allowed to thaw and crystallize in the microgravity environment on Mir Space Station. A later crew will return the Dewar to Earth for sample analysis. Dr. Alexander McPherson of the University of California at Riverside is the principal investigator. Photo credit: NASA/Johnson Space Center.

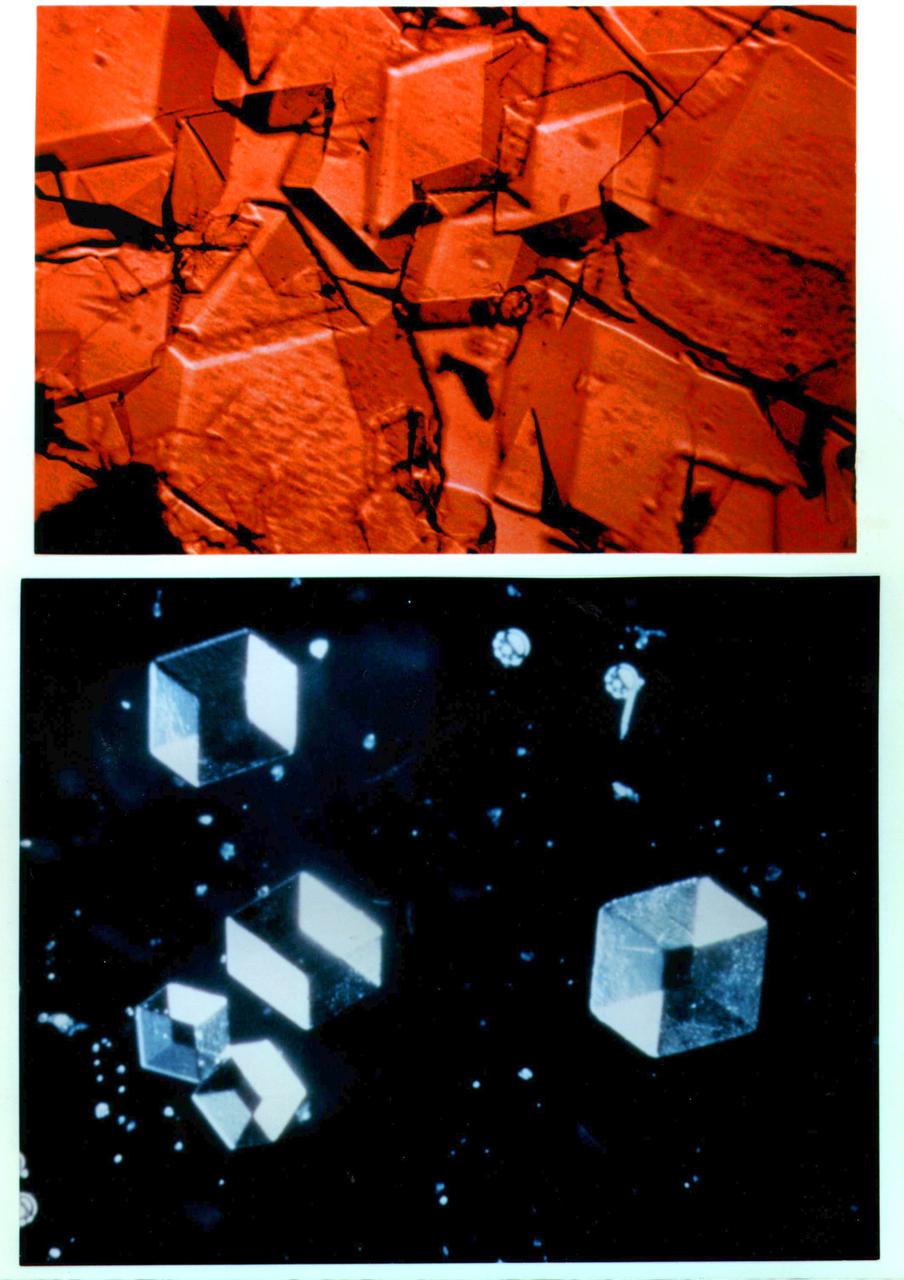
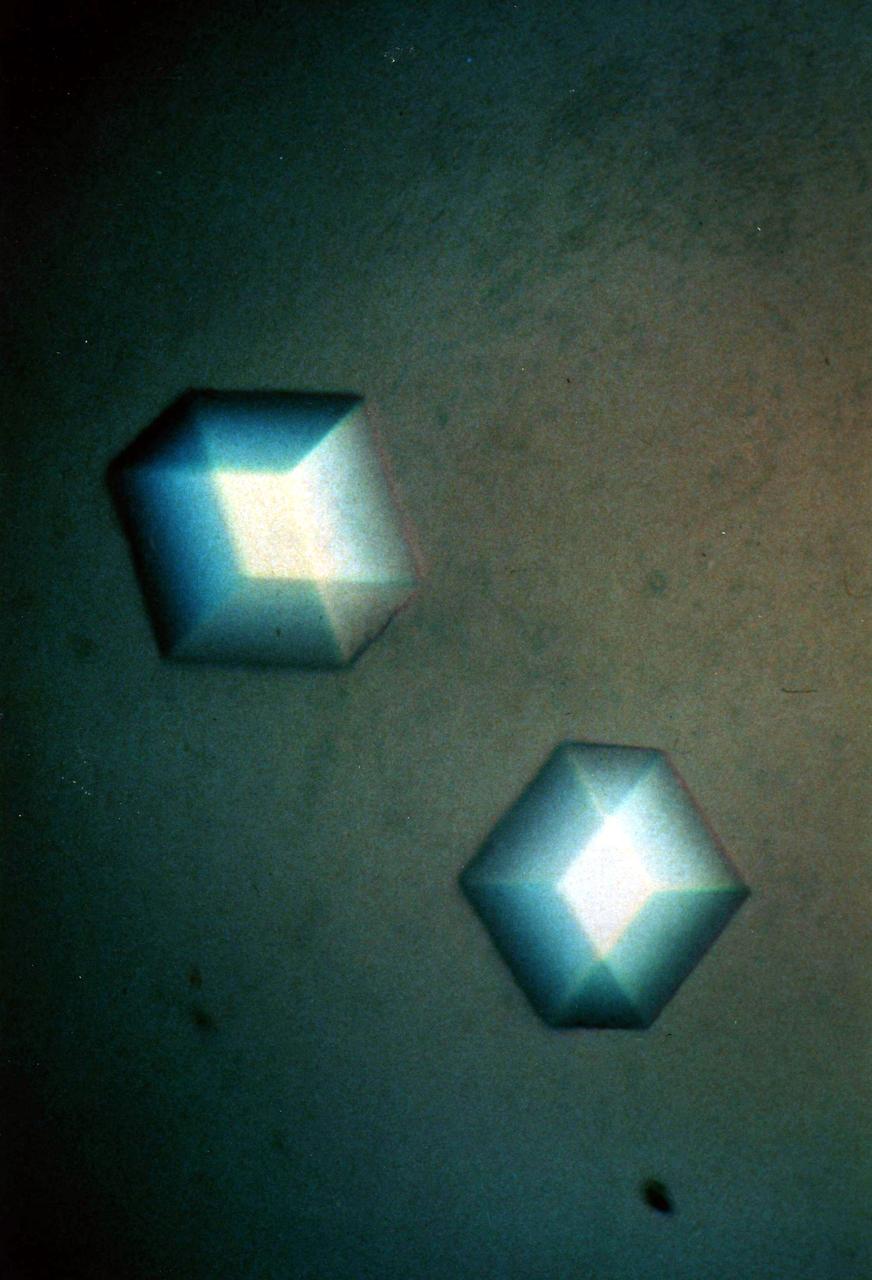
A collage of protein and virus crystals, many of which were grown on the U.S. Space Shuttle or Russian Space Station, Mir. The crystals include the proteins canavalin; mouse monoclonal antibody; a sweet protein, thaumatin; and a fungal protease. Viruses are represented here by crystals of turnip yellow mosaic virus and satellite tobacco mosaic virus. The crystals are photographed under polarized light (thus causing the colors) and range in size from a few hundred microns in edge length up to more than a millimeter. All the crystals are grown from aqueous solutions and are useful for X-ray diffraction analysis. Credit: Dr. Alex McPherson, University of California, Irvine.

High school students screen crystals of various proteins that are part of the ground-based work that supports Alexander McPherson's protein crystal growth experiment. The students also prepared and stored samples in the Enhanced Gaseous Nitrogen Dewar, which was launched on the STS-98 mission for delivery to the ISS. The crystals grown on the ground will be compared with crystals grown in orbit. Participants include Joseph Negron, of Terry Parker High School, Jacksonville, Florida; Megan Miskowski (shown), of Ridgeview High School, Orange Park, Florida; and Sam Swank, of Fletcher High School, Neptune Beach, Florida. The proteins are placed in plastic tubing that is heat-sealed at the ends, then flash-frozen and preserved in a liquid nitrogen Dewar. Aboard the ISS, the nitrogen will be allowed to evaporated so the samples thaw and then slowly crystallize. They will be analyzed after return to Earth. Photo credit: NASA/Marshall Space Flight Center.
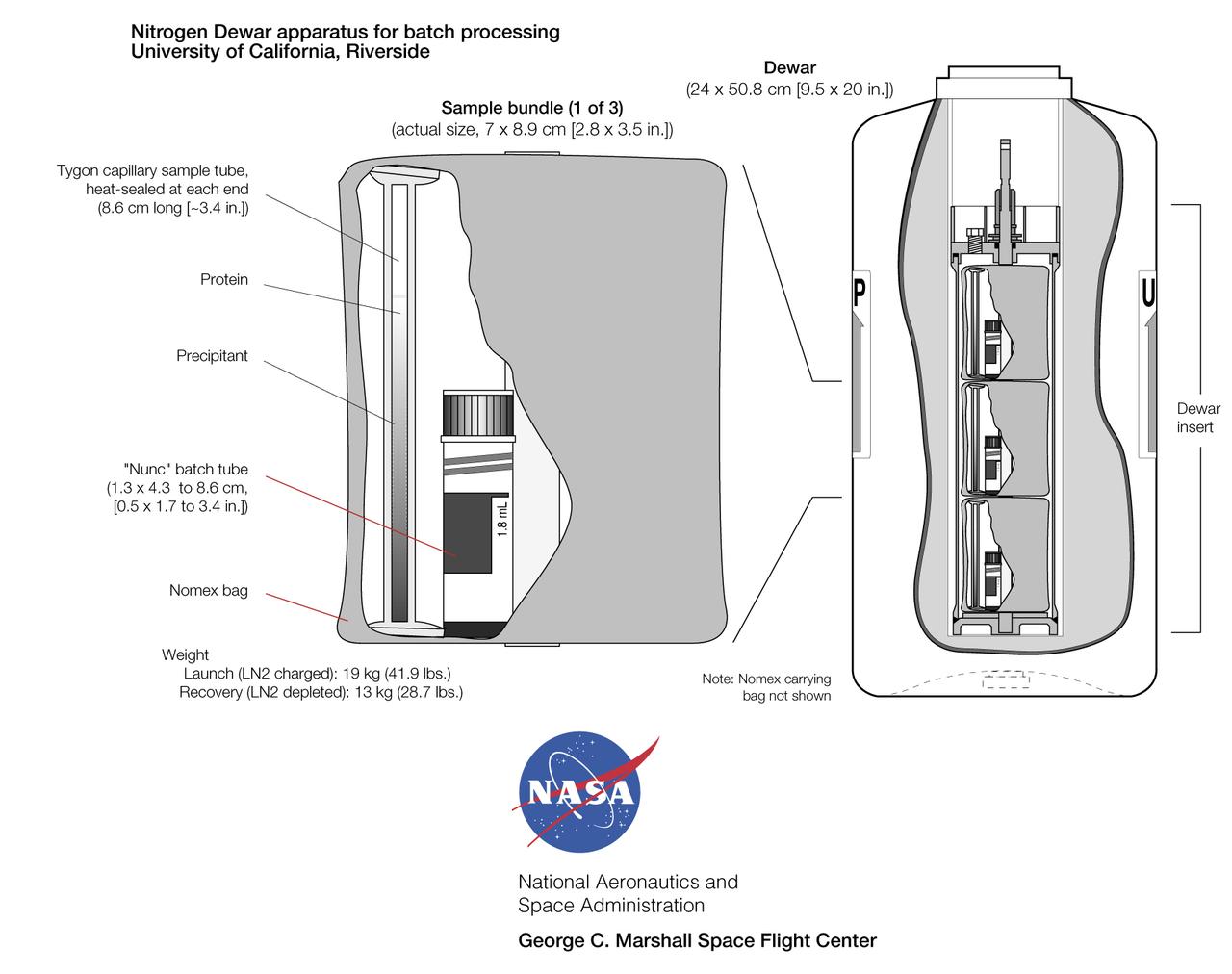
Gaseous Nitrogen Dewar apparatus developed by Dr. Alex McPherson of the University of California, Irvine for use aboard Mir and the International Space Station allows large quantities of protein samples to be crystallized in orbit. The specimens are contained either in plastic tubing (heat-sealed at each end). Biological samples are prepared with a precipitating agent in either a batch or liquid-liquid diffusion configuration. The samples are then flash-frozen in liquid nitrogen before crystallization can start. On orbit, the Dewar is placed in a quiet area of the station and the nitrogen slowly boils off (it is taken up by the environmental control system), allowing the proteins to thaw to begin crystallization. The Dewar is returned to Earth after one to four months on orbit, depending on Shuttle flight opportunities. The tubes then are analyzed for crystal presence and quality
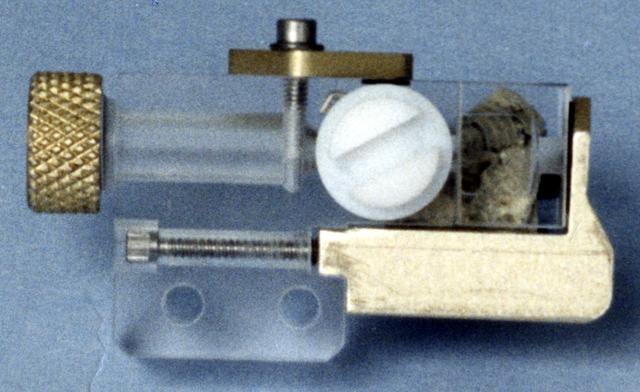
This photo shows an individual cell from the Handheld Diffusion Test Cell (HH-DTC) apparatus flown on the Space Shuttle. Similar cells will be used in the Observable Protein Crystal Growth Apparatus (OPCGA) to be operated aboard the International Space Station (ISS). The principal investigator is Dr. Alex McPherson of the University of California, Irvine. Each individual cell comprises two sample chambers with a rotating center section that isolates the two from each other until the start of the experiment and after it is completed. The cells are made from optical-quality quartz glass to allow photography and interferometric observations. Each cell has a small light-emitting diode and lens to back-light the solution. In protein crystal growth experiments, a precipitating agent such as a salt solution is used to absorb and hold water but repel the protein molecules. This increases the concentration of protein until the molecules nucleate to form crystals. This cell is one of 96 that make up the experiment module portion of the OPCGA.

High school students screen crystals of various proteins that are part of the ground-based work that supports Alexander McPherson's protein crystal growth experiment. The students also prepared and stored samples in the Enhanced Gaseous Nitrogen Dewar, which was launched on the STS-98 mission for delivery to the ISS. The crystals grown on the ground will be compared with crystals grown in orbit. Participants include Joseph Negron, of Terry Parker High School, Jacksonville, Florida; Megan Miskowski, of Ridgeview High School, Orange Park, Florida; and Sam Swank (shown), of Fletcher High School, Neptune Beach, Florida. The proteins are placed in plastic tubing that is heat-sealed at the ends, then flash-frozen and preserved in a liquid nitrogen Dewar. Aboard the ISS, the nitrogen will be allowed to evaporated so the samples thaw and then slowly crystallize. They will be analyzed after return to Earth. Photo credit: NASA/Marshall Space Flight Center.
This photo shows the Handheld Diffusion Test Cell (HH-DTC) apparatus flown on the Space Shuttle. Similar cells (inside the plastic box) will be used in the Observable Protein Crystal Growth Apparatus (OPCGA) to be operated aboard the International Space Station (ISS). The principal investigator is Dr. Alex McPherson of the University of California, Irvine. Each individual cell comprises two sample chambers with a rotating center section that isolates the two from each other until the start of the experiment and after it is completed. The cells are made from optical-quality quartz glass to allow photography and interferometric observations. Each cell has a small light-emitting diode and lens to back-light the solution. In protein crystal growth experiments, a precipitating agent such as a salt solution is used to absorb and hold water but repel the protein molecules. This increases the concentration of protein until the molecules nucleate to form crystals. This cell is one of 96 that make up the experiment module portion of the OPCGA.

High school students screen crystals of various proteins that are part of the ground-based work that supports Alexander McPherson's protein crystal growth experiment. The students also prepared and stored samples in the Enhanced Gaseous Nitrogen Dewar, which was launched on the STS-98 mission for delivery to the ISS. The crystals grown on the ground will be compared with crystals grown in orbit. Participants include Joseph Negron (shown), of Terry Parker High School, Jacksonville, Florida; Megan Miskowski, of Ridgeview High School, Orange Park, Florida; and Sam Swank, of Fletcher High School, Neptune Beach, Florida. The proteins are placed in plastic tubing that is heat-sealed at the ends, then flash-frozen and preserved in a liquid nitrogen Dewar. Aboard the ISS, the nitrogen will be allowed to evaporated so the samples thaw and then slowly crystallize. They will be analyzed after return to Earth. Photo credit: NASA/Marshall Space Flight Center.
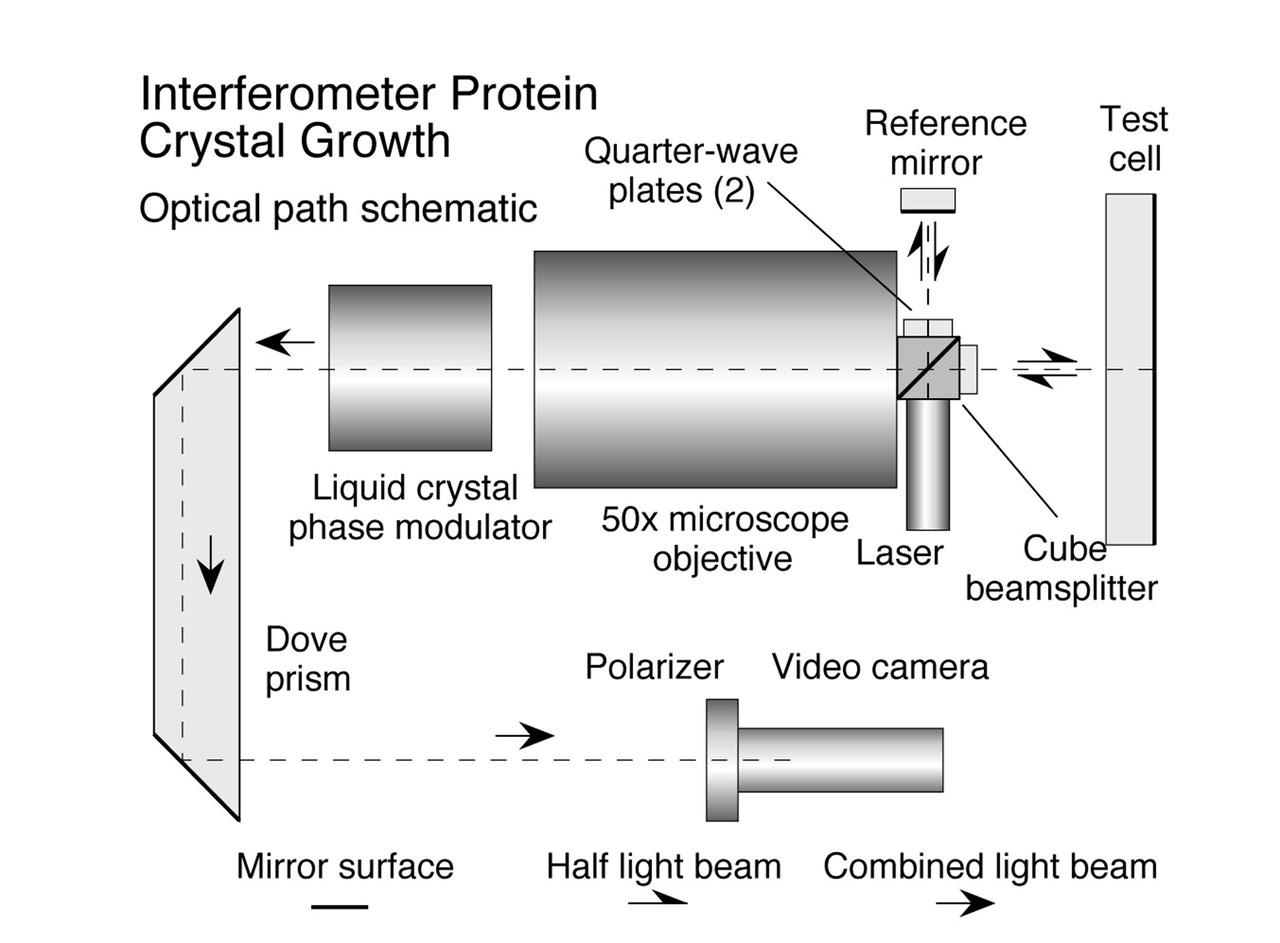
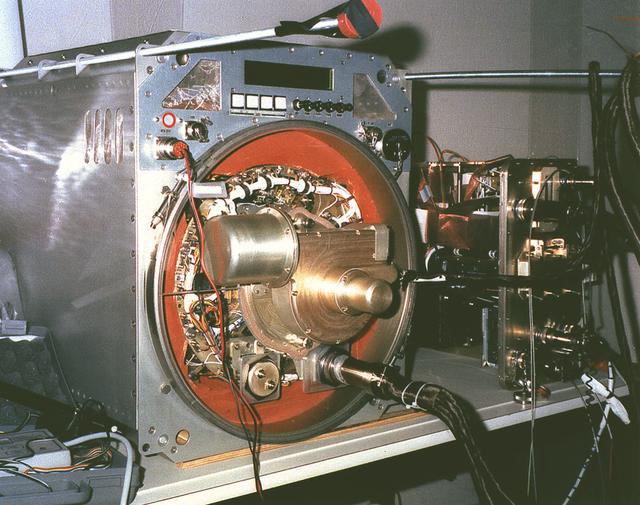
The Interferometer Protein Crystal Growth (IPCG) experiment was designed to measure details of how protein molecules move through a fluid. It was flown on the STS-86 mission for use aboard Russian Space Station Mir in 1998. It studied aspects of how crystals grow - and what conditions lead to the best crystals, details that remain a mystery. IPCG produces interference patterns by spilitting then recombining laser light. This let scientists see how fluid densities - and molecular diffusion - change around a crystal as it grows in microgravity. The heart of the IPCG apparatus is the interferometer cell comprising the optical bench, microscope, other optics, and video camera. IPCG experiment cells are made of optical glass and silvered on one side to serve as a mirror in the interferometer system that visuzlizes crystals and conditions around them as they grow inside the cell. This diagram shows the optical layout. The principal investigator was Dr. Alexander McPherson of University of California, Irvine. Co-investigators are William Witherow and Dr. Marc Pusey of NASA's Marshall Space Flight Center (MSFC).
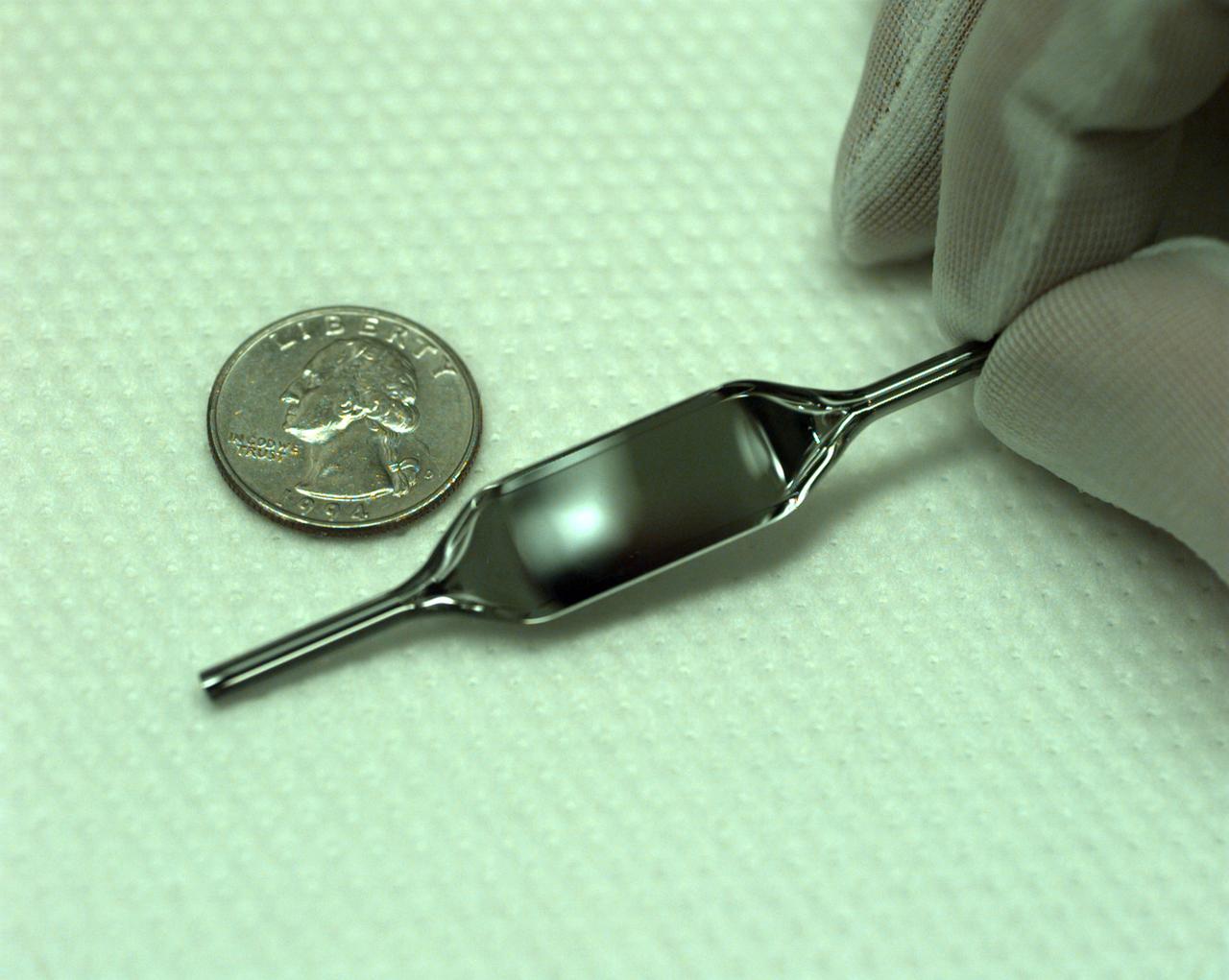
The Interferometer Protein Crystal Growth (IPCG) experiment was designed to measure details of how protein molecules move through a fluid. It was flown on the STS-86 mission for use aboard Russian Space Station Mir in 1998. It studied aspects of how crystals grow - and what conditions lead to the best crystals, details that remain a mystery. IPCG produces interference patterns by spilitting then recombining laser light. This let scientists see how fluid densities - and molecular diffusion - change around a crystal as it grows in microgravity. The heart of the IPCG apparatus is the interferometer cell comprising the optical bench, microscope, other optics, and video camera. IPCG experiment cells are made of optical glass and silvered on one side to serve as a mirror in the interferometer system that visuzlizes crystals and conditions around them as they grow inside the cell. This view shows a large growth cell. The principal investigator was Dr. Alexander McPherson of University of California, Irvine. Co-investigators are William Witherow and Dr. Marc Pusey of NASA's Marshall Space Flight Center (MSFC).
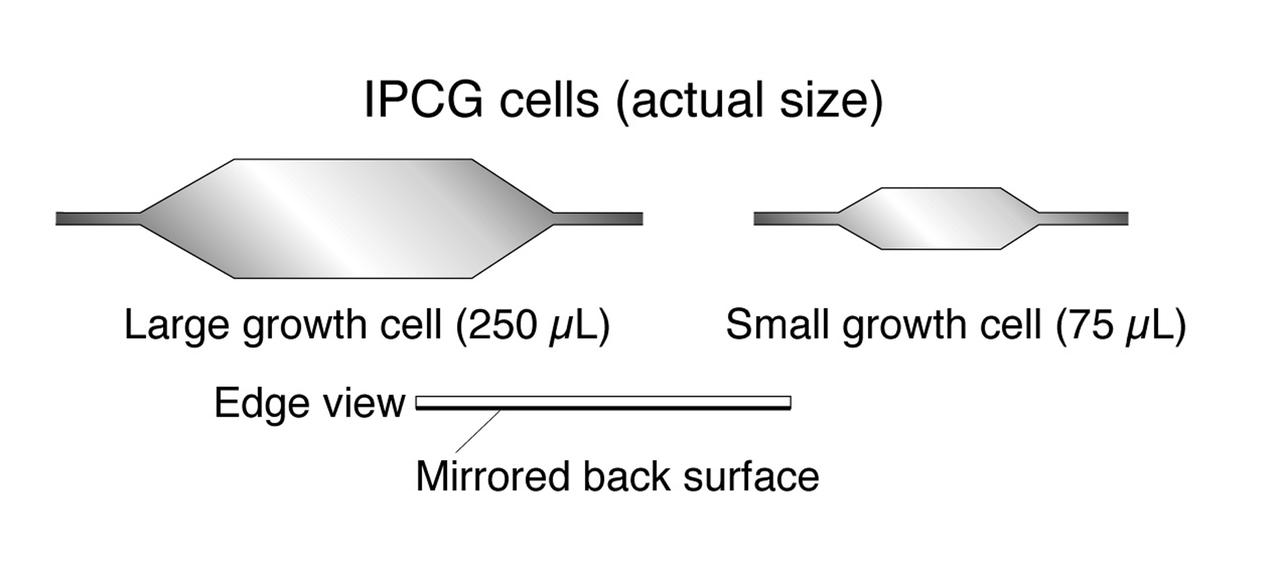
The Interferometer Protein Crystal Growth (IPCG) experiment was designed to measure details of how protein molecules move through a fluid. It was flown on the STS-86 mission for use aboard Russian Space Station Mir in 1998. It studied aspects of how crystals grow - and what conditions lead to the best crystals, details that remain a mystery. IPCG produces interference patterns by spilitting then recombining laser light. This let scientists see how fluid densities - and molecular diffusion - change around a crystal as it grows in microgravity. The heart of the IPCG apparatus is the interferometer cell comprising the optical bench, microscope, other optics, and video camera. IPCG experiment cells are made of optical glass and silvered on one side to serve as a mirror in the interferometer system that visuzlizes crystals and conditions around them as they grow inside the cell. This diagram shows the growth cells. The principal investigator was Dr. Alexander McPherson of University of California, Irvine. Co-investigators are William Witherow and Dr. Marc Pusey of NASA's Marshall Space Flight Center (MSFC).
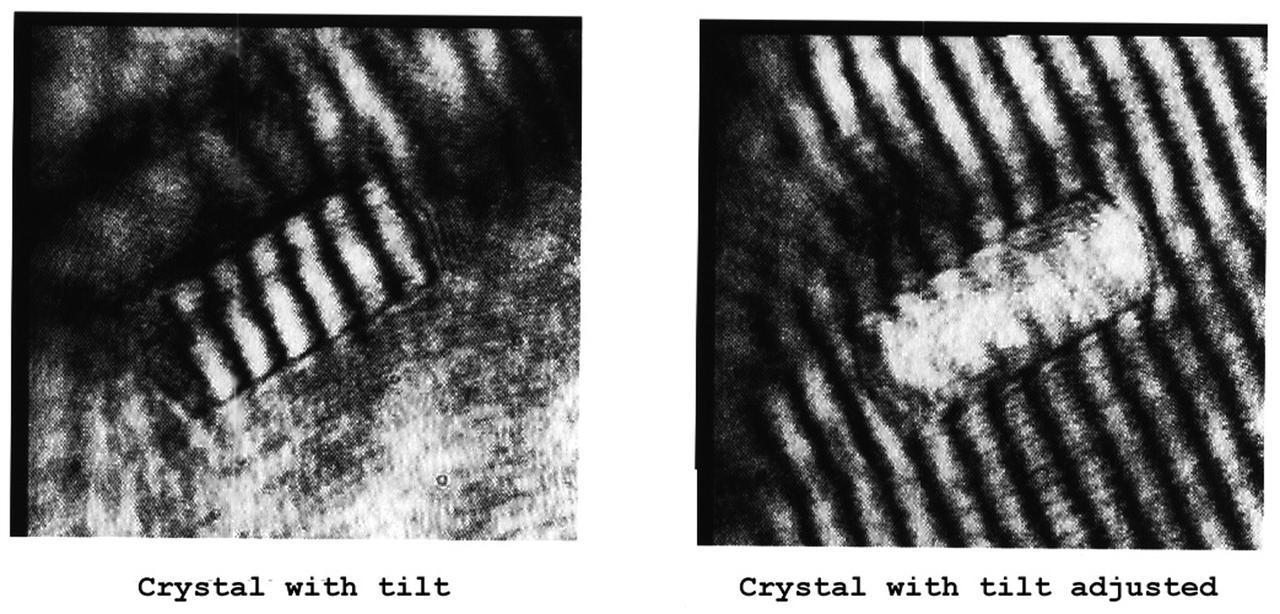
The Interferometer Protein Crystal Growth (IPCG) experiment was designed to measure details of how protein molecules move through a fluid. It was flown on the STS-86 mission for use aboard Russian Space Station Mir in 1998. It studied aspects of how crystals grow - and what conditions lead to the best crystals, details that remain a mystery. IPCG produces interference patterns by spilitting then recombining laser light. This let scientists see how fluid densities - and molecular diffusion - change around a crystal as it grows in microgravity. The heart of the IPCG apparatus is the interferometer cell comprising the optical bench, microscope, other optics, and video camera. IPCG experiment cells are made of optical glass and silvered on one side to serve as a mirror in the interferometer system that visuzlizes crystals and conditions around them as they grow inside the cell. This view shows interferograms produced in ground tests. The principal investigator was Dr. Alexander McPherson of University of California, Irvine. Co-investigators are William Witherow and Dr. Marc Pusey of NASA's Marshall Space Flight Center (MSFC).
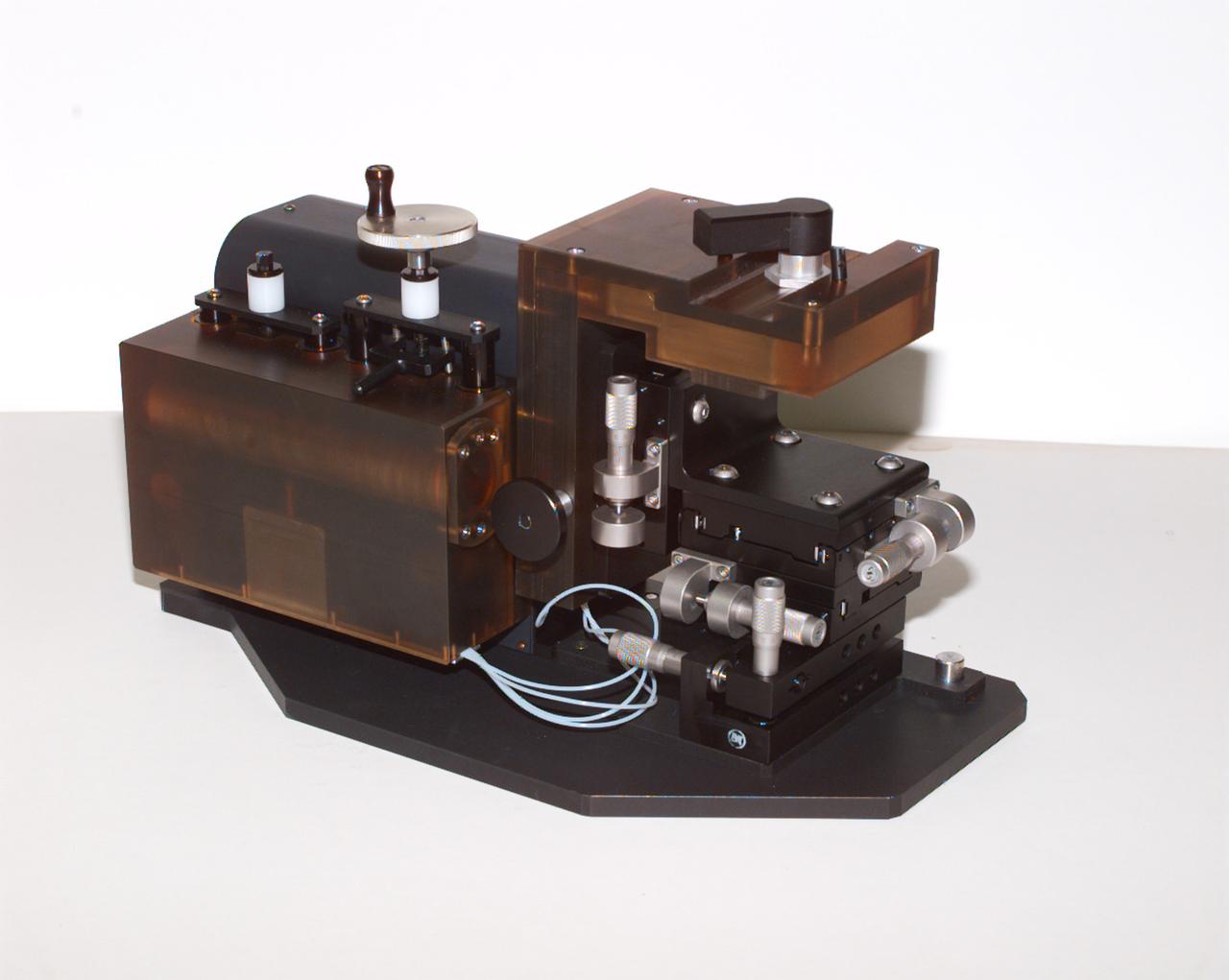
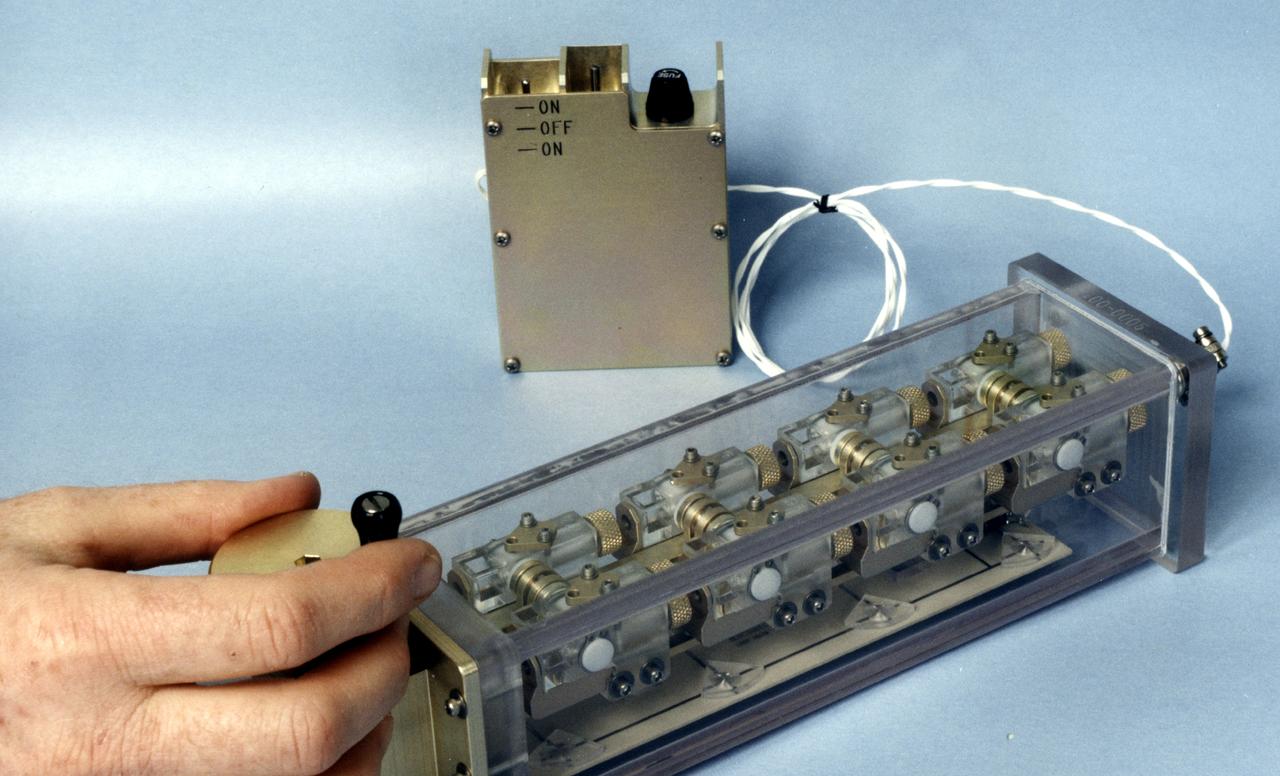
The Interferometer Protein Crstal Growth (IPCG) experiment was designed to measure details of how protein molecules move through a fluid. It was flown on the STS-86 mission for use aboard Russin Space Station Mir in 1998. It studied aspects of how crystals grow - and what conditions lead to the best crystals, details that remain a mystery. IPCG produces interference patterns by splitting then recombining laser light. This let scientists see how fluid densities - and molecular diffusion - change around a crystal as it grows in microgravity. The heart of the IPCG apparatus is the interferometer cell comprising the optical bench, microscope, other optics, and video camera. IPCG experiment cells are made of optical glass and silvered on one side to serve as a mirror in the interferometer system that visualizes crystals and conditions around them as they grow inside the cell. This view shows the complete apparatus. The principal investigator was Dr. Alexander McPherson of the University of California, Irvin. Co-investigators are William Witherow and Dr. Marc Pusey of NASA's Marshall Space Flight Center

Visit to GRC by the Deputy Administrator, James Morhard

Visit to GRC by the Deputy Administrator, James Morhard

CAPE CANAVERAL, Fla. -- In the high bay of the RTG storage facility at NASA's Kennedy Space Center in Florida, Innovative Health Applications employee Mike McPherson measures the level of radioactivity emitted from the multi-mission radioisotope thermoelectric generator (MMRTG) for NASA's Mars Science Laboratory mission, enclosed in a shipping cask at right. The MMRTG will generate the power needed for the mission from the natural decay of plutonium-238, a non-weapons-grade form of the radioisotope. Heat given off by this natural decay will provide constant power through the day and night during all seasons. Waste heat from the MMRTG will be circulated throughout the rover system to keep instruments, computers, mechanical devices and communications systems within their operating temperature ranges. MSL's components include a compact car-sized rover, Curiosity, which has 10 science instruments designed to search for evidence on whether Mars has had environments favorable to microbial life, including chemical ingredients for life. The unique rover will use a laser to look inside rocks and release its gasses so that the rover’s spectrometer can analyze and send the data back to Earth. Launch of MSL aboard a United Launch Alliance Atlas V rocket is scheduled for Nov. 25 from Space Launch Complex 41 on Cape Canaveral Air Force Station in Florida. For more information, visit http://www.nasa.gov/msl. Photo credit: NASA/Frankie Martin

Visit to GRC by the Deputy Administrator, James Morhard