
Dr. Marc Pusey (seated) and Dr. Craig Kundrot use computers to analyze x-ray maps and generate three-dimensional models of protein structures. With this information, scientists at Marshall Space Flight Center can learn how proteins are made and how they work. The computer screen depicts a proten structure as a ball-and-stick model. Other models depict the actual volume occupied by the atoms, or the ribbon-like structures that are crucial to a protein's function.

Cindy Barnes of University Space Research Association (USRA) at NASA's Marshall Space Flight Center pipettes a protein solution in preparation to grow crystals as part of NASA's structural biology program. Research on Earth helps scientists define conditions and specimens they will use in space experiments.

Edward Snell, a National Research Council research fellow at NASA's Marshall Space Flight Center (MSFC), prepares a protein crystal for analysis by x-ray crystallography as part of NASA's structural biology program. The small, individual crystals are bombarded with x-rays to produce diffraction patterns, a map of the intensity of the x-rays as they reflect through the crystal.

iss057e114766 (12/9/2018) --- European Space Agency (ESA) astronaut Alex Gerst is photographed with the Crystallization of RAS in Space (CASIS PCG 17) investigation. CASIS PCG 17grows crystals of KRAS proteins, which have a pivotal role in cell growth and death. Mutations in KRAS proteins are responsible for a third of all cancers and identifying the structure of these proteins is critical to developing therapeutics and treatments. Protein crystals grow larger and more perfectly in microgravity, allowing for detailed laboratory analysis of their structure back on Earth.

iss057e114765 (12/9/2018) --- European Space Agency (ESA) astronaut Alex Gerst is photographed with the Crystallization of RAS in Space (CASIS PCG 17) investigation. CASIS PCG 17grows crystals of KRAS proteins, which have a pivotal role in cell growth and death. Mutations in KRAS proteins are responsible for a third of all cancers and identifying the structure of these proteins is critical to developing therapeutics and treatments. Protein crystals grow larger and more perfectly in microgravity, allowing for detailed laboratory analysis of their structure back on Earth.
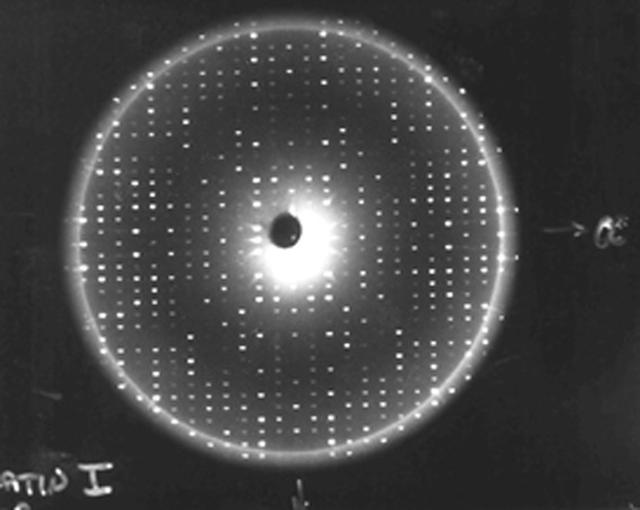
X-rays diffracted from a well-ordered protein crystal create sharp patterns of scattered light on film. A computer can use these patterns to generate a model of a protein molecule. To analyze the selected crystal, an X-ray crystallographer shines X-rays through the crystal. Unlike a single dental X-ray, which produces a shadow image of a tooth, these X-rays have to be taken many times from different angles to produce a pattern from the scattered light, a map of the intensity of the X-rays after they diffract through the crystal. The X-rays bounce off the electron clouds that form the outer structure of each atom. A flawed crystal will yield a blurry pattern; a well-ordered protein crystal yields a series of sharp diffraction patterns. From these patterns, researchers build an electron density map. With powerful computers and a lot of calculations, scientists can use the electron density patterns to determine the structure of the protein and make a computer-generated model of the structure. The models let researchers improve their understanding of how the protein functions. They also allow scientists to look for receptor sites and active areas that control a protein's function and role in the progress of diseases. From there, pharmaceutical researchers can design molecules that fit the active site, much like a key and lock, so that the protein is locked without affecting the rest of the body. This is called structure-based drug design.

Eddie Snell (standing), Post-Doctoral Fellow the National Research Council (NRC),and Marc Pusey of Marshall Space Flight Center (MSFC) use a reciprocal space mapping diffractometer for marcromolecular crystal quality studies. The diffractometer is used in mapping the structure of marcromolecules such as proteins to determine their structure and thus understand how they function with other proteins in the body. This is one of several analytical tools used on proteins crystalized on Earth and in space experiments. Photo credit: NASA/Marshall Space Flight Center (MSFC)
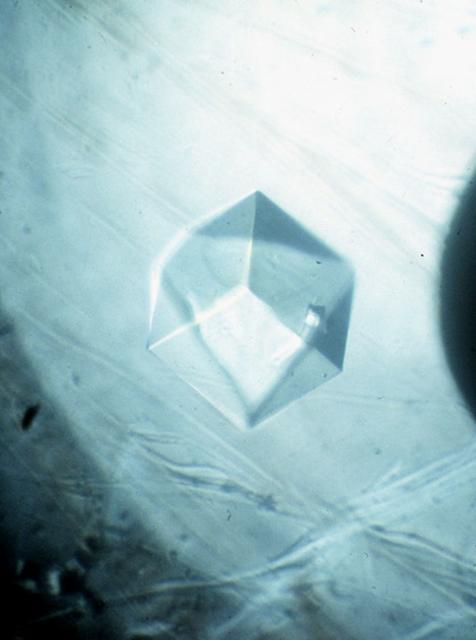
Malic Enzyme is a target protein for drug design because it is a key protein in the life cycle of intestinal parasites. After 2 years of effort on Earth, investigators were unable to produce any crystals that were of high enough quality and for this reason the structure of this important protein could not be determined. Crystals obtained from one STS-50 were of superior quality allowing the structure to be determined. This is just one example why access to space is so vital for these studies. Principal Investigator is Larry DeLucas.
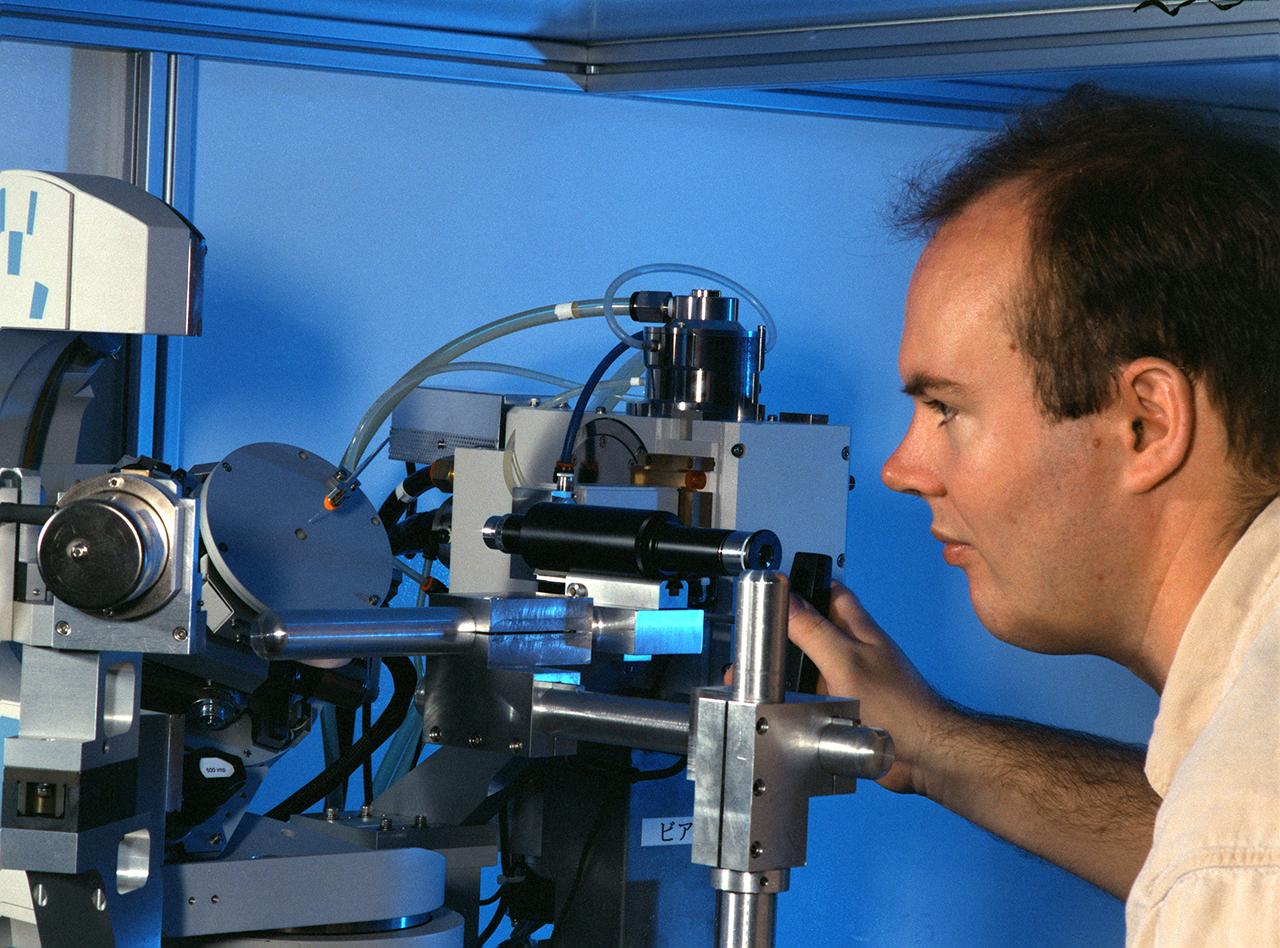
Eddie Snell, Post-Doctoral Fellow the National Research Council (NRC) uses a reciprocal space mapping diffractometer for macromolecular crystal quality studies. The diffractometer is used in mapping the structure of macromolecules such as proteins to determine their structure and thus understand how they function with other proteins in the body. This is one of several analytical tools used on proteins crystallized on Earth and in space experiments. Photo credit: NASA/Marshall Space Flight Center (MSFC)
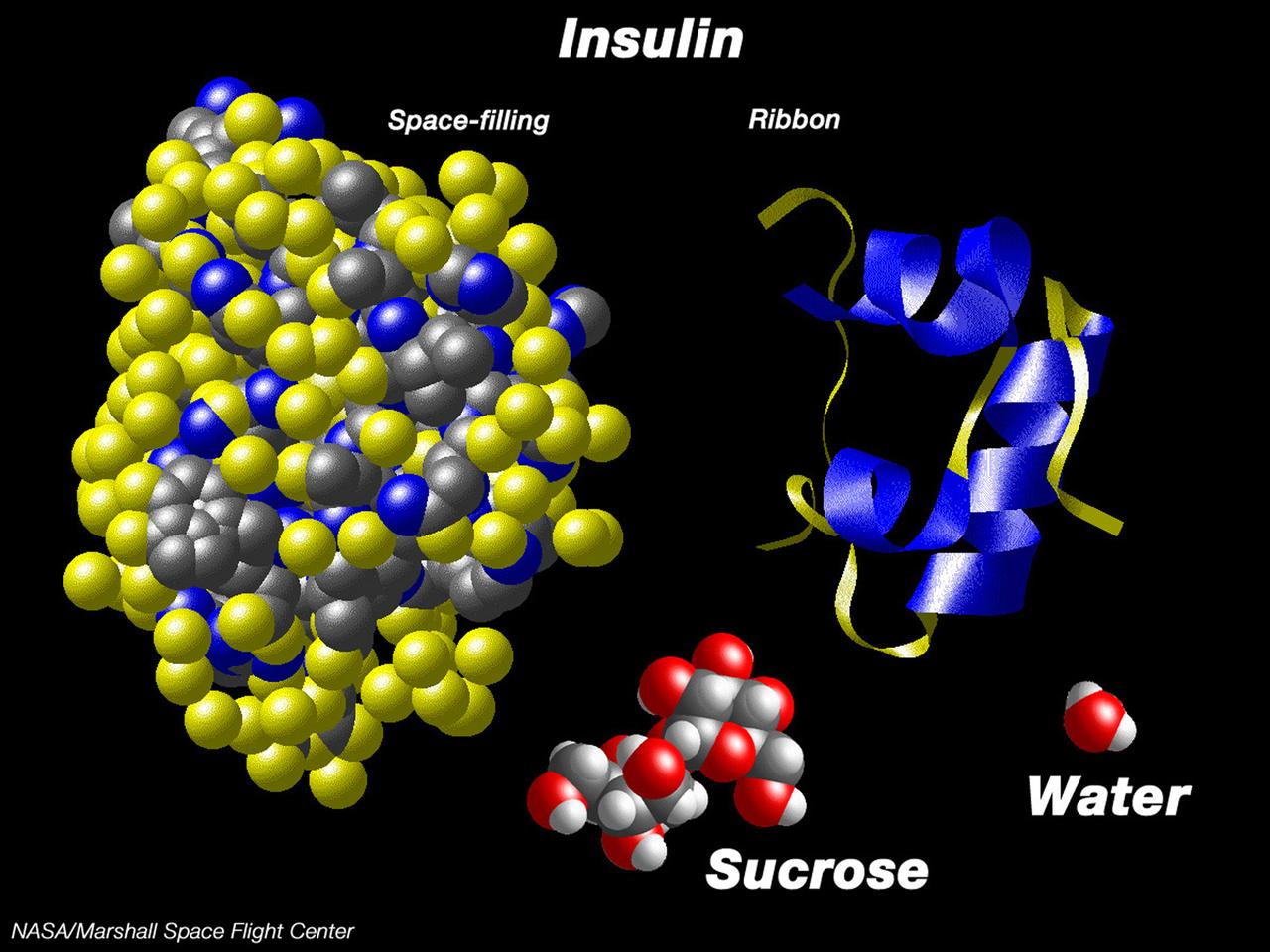
This computer graphic depicts the relative complexity of crystallizing large proteins in order to study their structures through x-ray crystallography. Insulin is a vital protein whose structure has several subtle points that scientists are still trying to determine. Large molecules such as insuline are complex with structures that are comparatively difficult to understand. For comparison, a sugar molecule (which many people have grown as hard crystals in science glass) and a water molecule are shown. These images were produced with the Macmolecule program. Photo credit: NASA/Marshall Space Flight Center (MSFC)
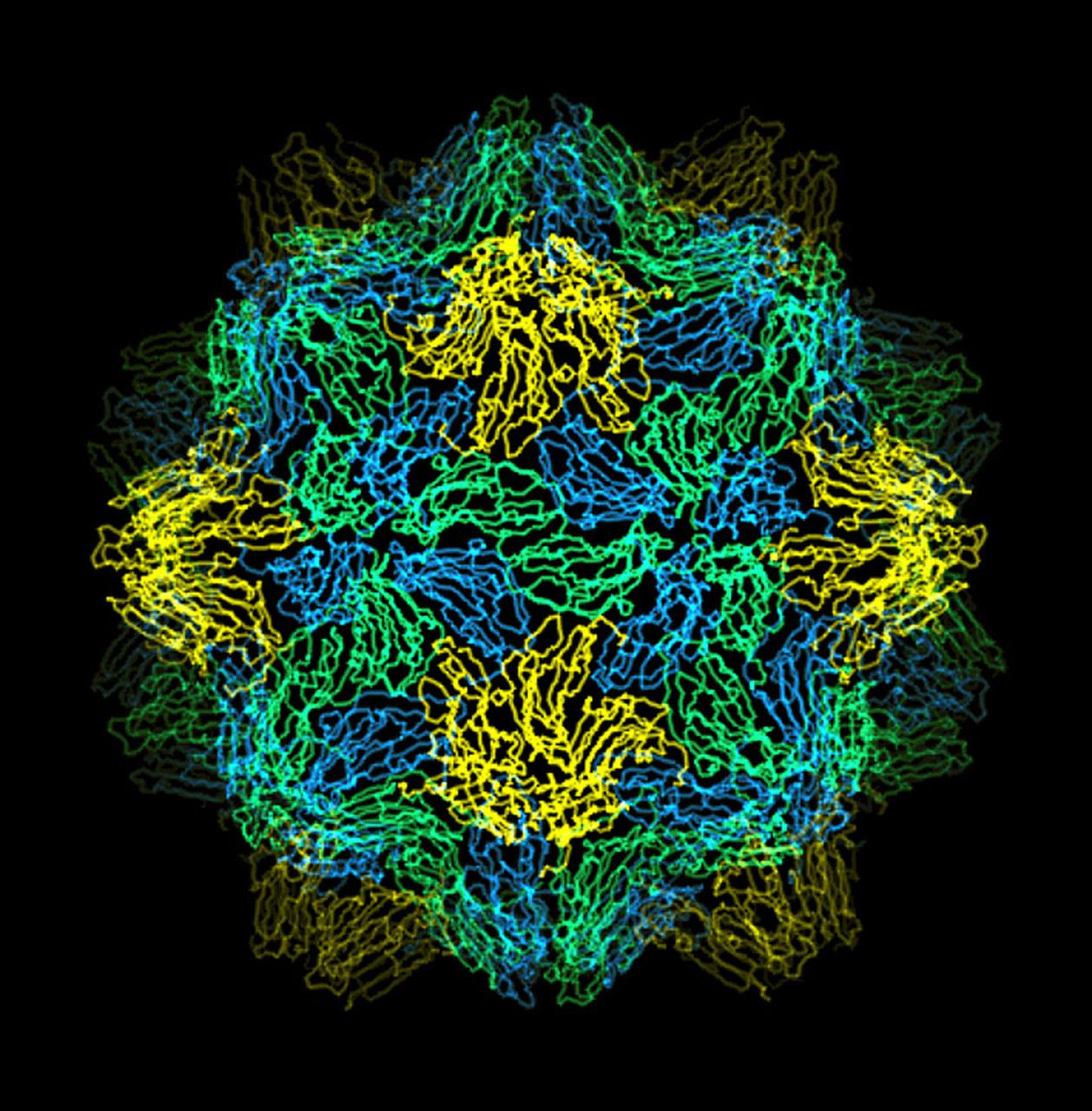
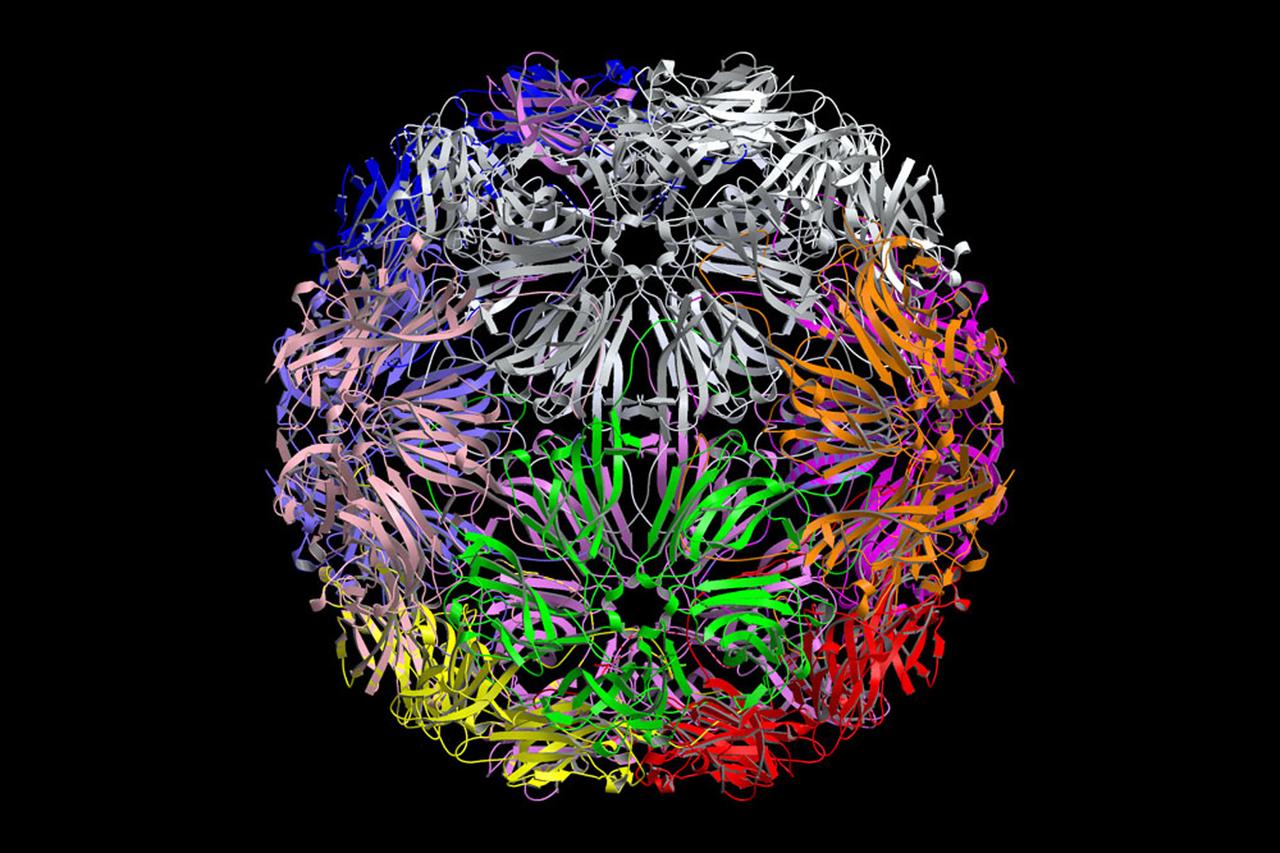
The bumpy exterior of the turnip yellow mosaic virus (TYMV) protein coat, or capsid, was defined in detail by Dr. Alexander McPherson of the University of California, Irvin using protein crystallized in space for analysis on Earth. TYMV is an icosahedral virus constructed from 180 copies of the same protein arranged into 12 clusters of five proteins (pentamers), and 20 clusters of six proteins (hexamers). The final TYMV structure led to the enexpected hypothesis that the virus release its RNA by essentially chemical-mechanical means. Most viruses have farly flat coats, but in TYMV, the fold in each protein, called the jellyroll, is clustered at the points where the protein pentamers and hexamers join. The jellyrolls are almost standing on end, producing a bumpy surface with knobs at all of the pentamers and hexamers. At the inside surface of the pentamers is a void that is not present at the hexamers. The coating had been seen in early studies of TYMV, but McPhereson's atomic structure shows much more detail. The inside surface is strikingly, and unexpectedly, different than the outside. While the pentamers contain a central viod on the inside, the hexameric units contain peptides liked to each other, forming a ring or, more accurately, rings to fill the voild. Credit: Dr. Alexander McPherson, University of California, Irvine.

The bumpy exterior of the turnip yellow mosaic virus (TYMV) protein coat, or capsid, was defined in detail by Dr. Alexander McPherson of the University of California, Irvin using proteins crystallized in space for analysis on Earth. TYMV is an icosahedral virus constructed from 180 copies of the same protein arranged into 12 clusters of five proteins (pentamers), and 20 clusters of six proteins (hexamers). The final TYMV structure led to the unexpected hypothesis that the virus releases its RNA by essentially chemical-mechanical means. Most viruses have fairly flat coats, but in TYNV, the fold in each protein, called the jellyroll, is clustered at the points where the protein pentamers and hexamers join. The jellyrolls are almost standing on end, producing a bumpy surface with knobs at all of the pentamers and hexamers. At the inside surface of the pentamers is a void that is not present at the hexamers. The coating had been seen in early stuties of TYMV, but McPherson's atomic structure shows much more detail. The inside surface is strikingly, and unexpectedly, different than the outside. While the pentamers contain a central void on the inside, the hexameric units contain peptides linked to each other, forming a ring or, more accurately, rings to fill the void. Credit: Dr. Alexander McPherson, University of California, Irvine
Proteins are the building blocks of our bodies and the living world around us. Within our bodies proteins make it possible for red blood cells to carry oxygen throughout the body. Others help transmit nerve impulses so we can hear, smell and feel the world around us. While others play a crucial role in preventing or causing disease. If the structure of a protein is known, then companies can develop new or improved drugs to fight the disease of which the protein is a part. To determine protein structure, researchers must grow near-perfect crystals of the protein. On Earth convection currents, sedimentation and other gravity-induced phenomena hamper crystal growth efforts. In microgravity researchers can grow near-perfect crystals in an environment free of these effects. Because of the enormous potential for new pharmaceutical products the Center for Macromolecular Crystallography--the NASA Commercial Space Center responsible for commercial protein crystal growth efforts has more than fifty major industry and academic partners. Research on crystals of human insulin could lead to improved treatments for diabetes.

Dr. Laurel Karr of NASA's Marshall Space Flight Center uses a stereo microscope to analyz protein crystals as a part of NASA's structural biology program.

iss062e087808 (3/11/2020) --- A view of Protein Crystal Growth-10 experiment hardware inside JAXA's (Japan Aerospace Exploration Agency) Kibo laboratory module aboard the International Space Station (ISS). Microgravity Crystallization of Glycogen Synthase-Glycogenin Protein Complex (CASIS PCG 10) crystallizes human glycogen synthase proteins on the space station. Determining the structure of the human glycogen synthase and full-length glycogenin protein complex could facilitate the development of treatments on Earth for metabolic disorders such as Type 2 diabetes, obesity, rare genetic disorders, and some forms of cancer.

STS066-13-029 (3-14 Nov 1994) --- On the Space Shuttle Atlantis' mid-deck, astronaut Scott E. Parazynski, mission specialist, works at one of two areas onboard the Shuttle which support the Protein Crystal Growth (PCG) experiment. This particular section is called the Vapor Diffusion Apparatus (VDA), housed in a Single Locker Thermal Enclosure (STES). Together with the Crystal Observation System, housed in the Thermal Enclosure System (COS/TES) the VDA represents the continuing research into the structures of proteins and other macromolecules such as viruses. In addition to using the microgravity of space to grow high-quality protein crystals for structural analyses, the experiments are expected to help develop technologies and methods to improve the protein crystallization process on Earth as well as in space.

NASA scientist, in the Space Sciences lab at Marshall, works with capillary optics that generate more intense X-rays than conventional sources. This capability is useful in studying the structure of important proteins.

iss047e154711 (6/17/2016) --- Photographic documentation of Luch-2M Multipurpose Crystallization Cassette (УБК) within orange case. Struktura is a study of protein crystallization processes and growth of single crystals which are suitable for X-ray structural analysis and structural decoding.

iss060e015014 (7/28/2019) — NASA astronaut Nick Hague is shown holding the CASIS Protein Crystal Growth 15 (CASIS PCG 15) investigation samples aboard the International Space Station (ISS). Microgravity Crystal Growth for Improvement in Neutron Diffraction and the Analysis of Protein Complexes (CASIS PCG 15) seeks a better understanding of enzyme catalysis by examining crystals from two model Pyridoxal phosphate (PLP) dependent enzymes and from a bacteriophage transient deoxyribonucleic acid (DNA) repair complex. Analysis of the crystals may reveal catalyst mechanisms and structures and visualize the interaction between the repair proteins. Results could contribute to identification of biomarkers for diagnosis of disease and to development of better antimicrobials.

iss064e011644 (Dec. 10, 2020) --- JAXA (Japan Aerospace Exploration Agency) astronaut and Expedition 64 Flight Engineer Soichi Noguchi mixes and dispenses samples for a biomedical study that explores creating high quality protein crystals in space. Observations from the advanced space research may provide detailed information on protein structures to develop new drugs for diseases.

On the Space Shuttle Atlantis' mid-deck, astronaut Joseph R. Tanner, mission specialist, works at area amidst several lockers onboard the Shuttle which support the Protein Crystal Growth (PCG) experiment. This particular section is called the Crystal Observation System, housed in the Thermal Enclosure System (COS/TES). Together with the Vapor Diffusion Apparatus (VDA), housed in a Single Locker Thermal Enclosure (SLTES) which is out of frame, the Cos/TES represents the continuing research into the structures of proteins and other macromolecules such as viruses.

On the Space Shuttle Orbiter Atlantis' middeck, Astronaut Joseph R. Tarner, mission specialist, works at an area amidst several lockers which support the Protein Crystal Growth (PCG) experiment during the STS-66 mission. This particular section is called the Crystal Observation System, housed in the Thermal Enclosure System (COS/TES). Together with the Vapor Diffusion Apparatus (VDA), housed in Single Locker Thermal Enclosure (SLTES), the COS/TES represents the continuing research into the structure of proteins and other macromolecules such as viruses.

iss048e038163 (7/17/2016) --- Roscosmos cosmonaut Anatoly Ivanishin displays Luch-2M Multipurpose Crystallization Cassette (УБК) No. 3 during Struktura-Luch-2M (Structure-Beam-2M) experiment hardware activation and deployment. Image was taken in the Zvezda Service Module (SM) aboard the International Space Station (ISS). Struktura is a study of protein crystallization processes and growth of single crystals which are suitable for X-ray structural analysis and structural decoding.

iss048e038166 (7/19/2016) --- Photographic documentation of the Luch-2M Unit for the Struktura-Luch-2M (Structure-Beam-2M) experiment deployed on Panel 406 in the Zvezda Service Module. (SM) aboard the International Space Station (ISS). Struktura is a study of protein crystallization processes and growth of single crystals which are suitable for X-ray structural analysis and structural decoding.
iss048e038162 (7/19/2016) --- The hand of a crewmember displays Luch-2M Multipurpose Crystallization Cassette (УБК) No. 2 during Struktura-Luch-2M (Structure-Beam-2M) experiment hardware activation and deployment. Image was taken in the Zvezda Service Module (SM) aboard the International Space Station (ISS). Struktura is a study of protein crystallization processes and growth of single crystals which are suitable for X-ray structural analysis and structural decoding.

iss050e058807 (3/17/2017) --- A view of European Space Agency (ESA) astronaut Thomas Pesquet, during Protein Crystal Growth (PCG) -5 hardware deactivation and stow, from Microgravity Experiment Research Locker Incubator (MERLIN) on Expedite the Processing of Experiments to the Space Station (EXPRESS) Rack 5. The Microgravity Growth of Crystalline Monoclonal Antibodies for Pharmaceutical Applications (CASIS-PCG-5) investigation crystallizes a monoclonal antibody developed by Merck Research Labs. Microgravity enables the growth of extremely high-quality crystals, which allow scientists to study the proteins’ structure, improve drug delivery, manufacturing, and developing better methods for storing these biological molecules.

iss050e058812 (3/17/2017) --- A view of European Space Agency (ESA) astronaut Thomas Pesquet, during Protein Crystal Growth (PCG) -5 hardware deactivation and stow, from Microgravity Experiment Research Locker Incubator (MERLIN) on Expedite the Processing of Experiments to the Space Station (EXPRESS) Rack 5. The Microgravity Growth of Crystalline Monoclonal Antibodies for Pharmaceutical Applications (CASIS-PCG-5) investigation crystallizes a monoclonal antibody developed by Merck Research Labs. Microgravity enables the growth of extremely high-quality crystals, which allow scientists to study the proteins’ structure, improve drug delivery, manufacturing, and developing better methods for storing these biological molecules.

iss050e058802 (3/17/2017) --- A view of European Space Agency (ESA) astronaut Thomas Pesquet, during Protein Crystal Growth (PCG) -5 hardware deactivation and stow, from Microgravity Experiment Research Locker Incubator (MERLIN) on Expedite the Processing of Experiments to the Space Station (EXPRESS) Rack 5. The Microgravity Growth of Crystalline Monoclonal Antibodies for Pharmaceutical Applications (CASIS-PCG-5) investigation crystallizes a monoclonal antibody developed by Merck Research Labs. Microgravity enables the growth of extremely high-quality crystals, which allow scientists to study the proteins’ structure, improve drug delivery, manufacturing, and developing better methods for storing these biological molecules.

University of Alabama engineer Lance Weiss briefs NASA astronaut Dr. Bornie Dunbar about the design and capabilities of the X-ray Crystallography Facility under development at the Center for Macromolecular Crystallography of the University of Alabama at Birmingham, AL, April 21, 1999. The X-ray Crystallography Facility is designed to speed the collection of protein structure information from crystals grown aboard the International Space Station. By measuring and mapping the protein crystal structure in space, researchers will avoid exposing the delicate crystals to the rigors of space travel and make important research data available to scientists much faster. The X-ray Crystallography facility is being designed and developed by the Center for Macromolecular Crystallography of the University of Alabama at Birmingham, a NASA Commercial Space Center.

University of Alabama engineer Stacey Giles briefs NASA astronaut Dr. Bornie Dunbar about the design and capabilities of the X-ray Crystallography Facility under development at the Center for Macromolecular Crystallography of the University of Alabama at Birmingham, AL, April 21, 1999. The X-ray Crystallography Facility is designed to speed the collection of protein structure information from crystals grown aboard the International Space Station. By measuring and mapping the protein crystal structure in space, researchers will avoid exposing the delicate crystals to the rigors of space travel and make important research data available to scientists much faster. The X-ray Crystallography facility is being designed and developed by the Center for Macromolecular Crystallography of the University of Alabama at Birmingham, a NASA Commercial Space Center.
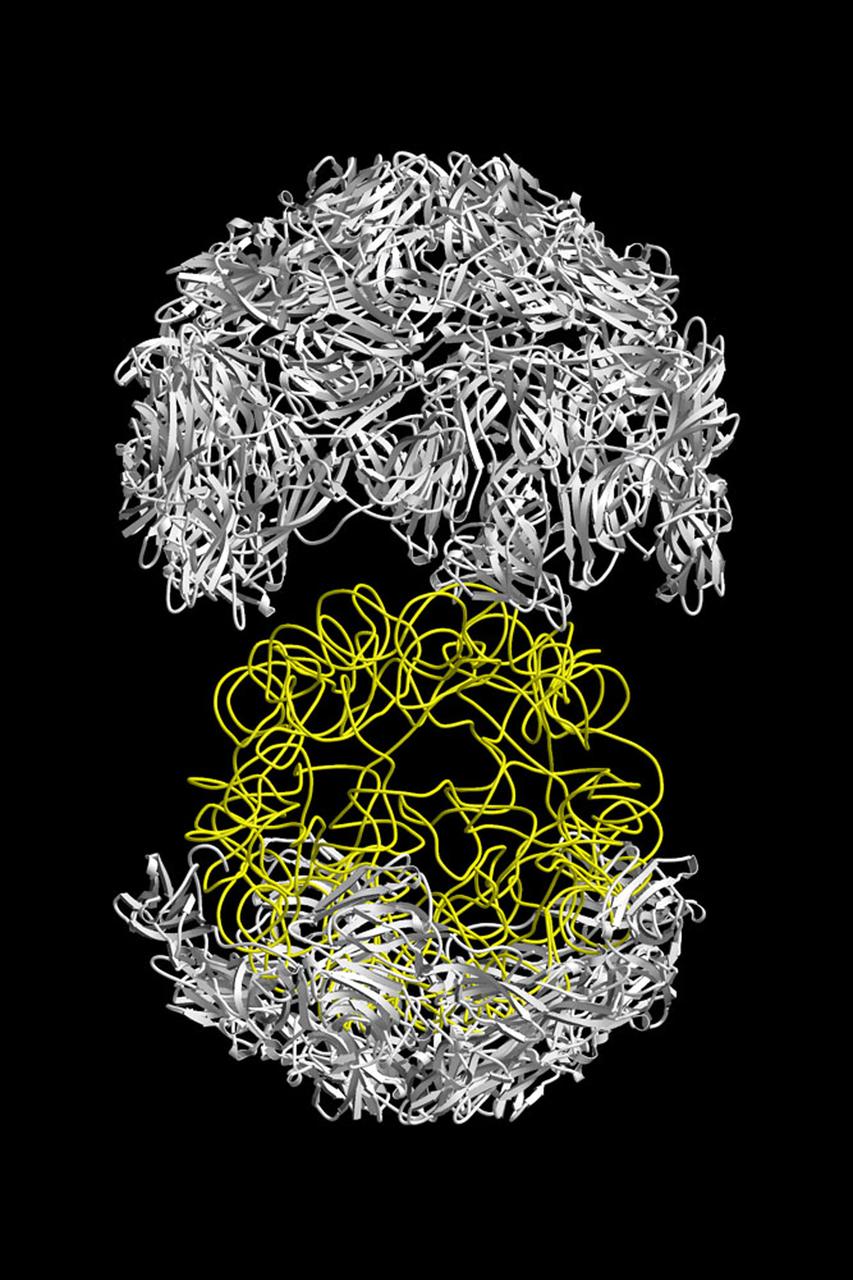
The structure of the Satellite Tobacco Mosaic Viurus (STMV)--one of the smallest viruses known--has been successfully reduced using STMV crystals grown aboard the Space Shuttle in 1992 and 1994. The STMV crystals were up to 30 times the volume of any seen in the laboratory. At the time they gave the best resolution data ever obtained on any virus crystal. STMV is a small icosahedral plant virus, consisting of a protein shell made up of 60 identical protein subunits of molecular weight 17,500. Particularly noteworthy is the fact that, in contrast to the crystals grown on Earth, the crystals grown under microgravity conditions were visually perfect, with no striations or clumping of crystals. Furthermore, the x-ray diffraction data obtained from the space-grown crystals was of a much higher quality than the best data available at that time from ground-based crystals. This stylized ribbon model shows the protein coat in white and the nucleic acid in yellow. STMV is used because it is a simple protein to work with; studies are unrelated to tobacco. Credit: Dr. Alex McPherson, University of California at Irvin.

iss055e010761 (4/5/2018) --- Photographic documentation of CASIS Protein Crystal Growth (PCG) -11 hardware during CS-DCB-Unpack2 activity aboard the International Space Station (ISS). Neutron Crystallographic Studies of Human Acetylcholinesterase for the Design of Accelerated Reactivators (CASIS PCG 11) produces acetylcholinesterase crystals, a neurotransmitter enzyme. Crystals grown in microgravity are larger, of higher-quality and can be used for a technique called macromolecular neutron crystallography (MNC) to locate hydrogen atoms in the crystal’s structure.

iss055e010753 (4/5/2018) --- Photographic documentation of CASIS Protein Crystal Growth (PCG) -11 hardware during CS-DCB-Unpack2 activity aboard the International Space Station (ISS). Neutron Crystallographic Studies of Human Acetylcholinesterase for the Design of Accelerated Reactivators (CASIS PCG 11) produces acetylcholinesterase crystals, a neurotransmitter enzyme. Crystals grown in microgravity are larger, of higher-quality and can be used for a technique called macromolecular neutron crystallography (MNC) to locate hydrogen atoms in the crystal’s structure.
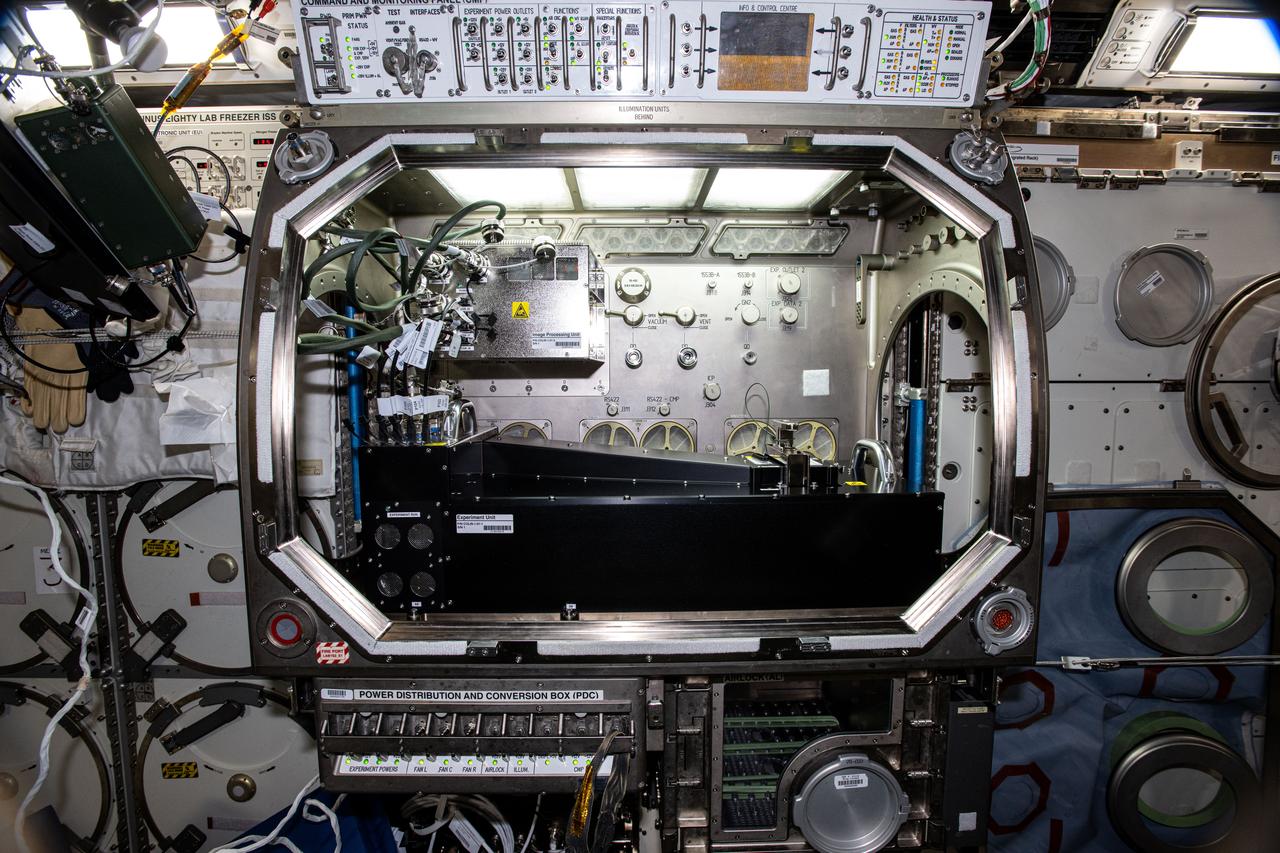
iss073e0002997 (4/28/2025) --- A view of the Colloidal Solids investigation inside the Microgravity Sciences Glovebox (MSG). Colloidal Solids (COLIS) provides researchers with a better understanding of the origin, formation, and dynamics of protein crystals and colloidal glasses and gels. COLIS is a state-of-the-art multi-line light scattering apparatus that enables the research team to monitor the dynamics of physical processes, during and after solidification, of soft matter solids on the International Space Station (ISS) to assess the role played by gravity on the properties of growing structures.
The structure of the Satellite Tobacco Mosaic Virus (STMV)--one of the smallest viruses known--has been successfully deduced using STMV crystals grown aboard the Space Shuttle in 1992 and 1994. The STMV crystals were up to 30 times the volume of any seen in the laboratory. At the same time they gave the best resolution data ever obtained on any virus crystal. STMV is a small icosahedral plant virus, consisting of a protein shell made up of 60 identical protein subunits of molecular weight 17,500. Particularly noteworthy is the fact that, in contrast to the crystal grown on Earth, the crystals grown under microgravity conditions were viusally perfect, with no striations or clumping of crystals. Furthermore, the X-ray diffraction data obtained from the space-grown crystals was of a much higher quality than the best data available at that time from ground-based crystals. This computer model shows the external coating or capsid. STMV is used because it is a simple protein to work with; studies are unrelated to tobacco. Credit: Dr. Alex McPherson, Univeristy of California at Irvin.
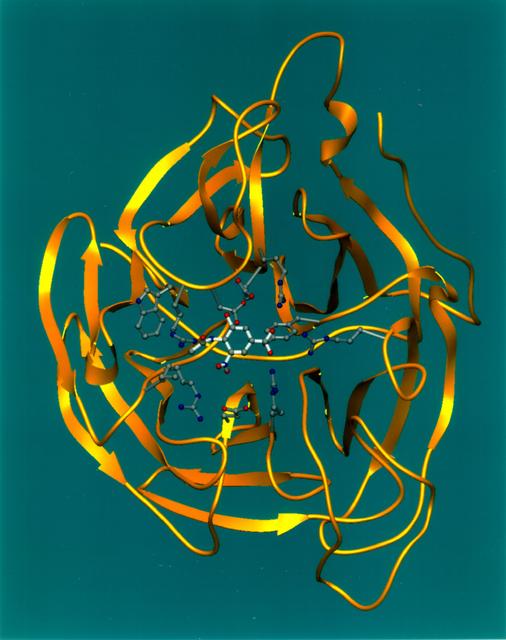
Ribbons is a program developed at UAB used worldwide to graphically depict complicated protein structures in a simplified format. The program uses sophisticated computer systems to understand the implications of protein structures. The Influenza virus remains a major causative agent for a large number of deaths among the elderly and young children and huge economic losses due to illness. Finding a cure will have a general impact both on the basic research of viral pathologists of fast evolving infectious agents and clinical treatment of influenza virus infection. The reproduction process of all strains of influenza are dependent on the same enzyme neuraminidase. Shown here is a segmented representation of the neuraminidase inhibitor compound sitting inside a cave-like contour of the neuraminidase enzyme surface. This cave-like formation present in every neuraminidase enzyme is the active site crucial to the flu's ability to infect. The space-grown crystals of neuraminidase have provided significant new details about the three-dimensional characteristics of this active site thus allowing researchers to design drugs that fit tighter into the site. Principal Investigator: Dr. Larry DeLucas

The Space Shuttle Columbia stands poised in the night for the STS-83 Microgravity Science Laboratory-1 (MSL-1) mission after the Rotating Service Structure of Launch Pad 39A has been moved back prior to the start of fueling operations that take place about 12 hours before liftoff. During the scheduled 16-day STS-83 mission, the MSL-1 will be used to test some of the hardware, facilities and procedures that are planned for use on the International Space Station as well as research in combustion, protein crystal growth and materials processing experiments

The Space Shuttle Columbia stands poised in the night for the STS-83 Microgravity Science Laboratory-1 (MSL-1) mission after the Rotating Service Structure of Launch Pad 39A has been moved back prior to the start of fueling operations that take place about 12 hours before liftoff. During the scheduled 16-day STS-83 mission, the MSL-1 will be used to test some of the hardware, facilities and procedures that are planned for use on the International Space Station as well as research in combustion, protein crystal growth and materials processing experiments

The Space Shuttle Columbia stands poised in the night for the STS-83 Microgravity Science Laboratory-1 (MSL-1) mission after the Rotating Service Structure of Launch Pad 39A has been moved back prior to the start of fueling operations that take place about 12 hours before liftoff. During the scheduled 16-day STS-83 mission, the MSL-1 will be used to test some of the hardware, facilities and procedures that are planned for use on the International Space Station as well as research in combustion, protein crystal growth and materials processing experiments

This is a Space Shuttle Columbia (STS-65) onboard photo of the second International Microgravity Laboratory (IML-2) in the cargo bay with Earth in the background. Mission objectives of IML-2 were to conduct science and technology investigations that required the low-gravity environment of space, with emphasis on experiments that studied the effects of microgravity on materials processes and living organisms. Materials science and life sciences are two of the most exciting areas of microgravity research because discoveries in these fields could greatly enhance the quality of life on Earth. If the structure of certain proteins can be determined by examining high-quality protein crystals grown in microgravity, advances can be made to improve the treatment of many human diseases. Electronic materials research in space may help us refine processes and make better products, such as computers, lasers, and other high-tech devices. The 14-nation European Space Agency (ESA), the Canadian Space Agency (SCA), the French National Center for Space Studies (CNES), the German Space Agency and the German Aerospace Research Establishment (DARA/DLR), and the National Space Development Agency of Japan (NASDA) participated in developing hardware and experiments for the IML missions. The missions were managed by NASA's Marshall Space Flight Center. The Orbiter Columbia was launched from the Kennedy Space Center on July 8, 1994 for the IML-2 mission.
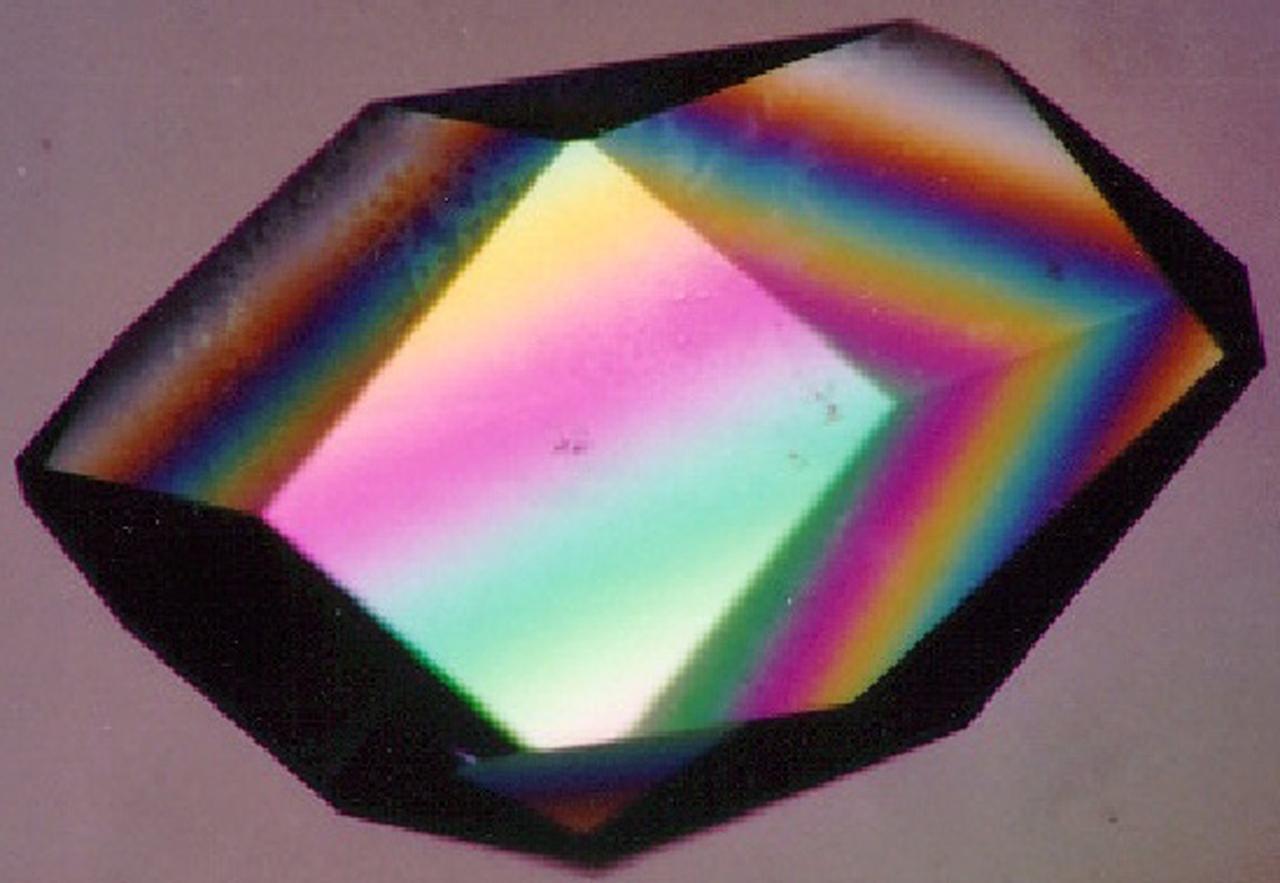
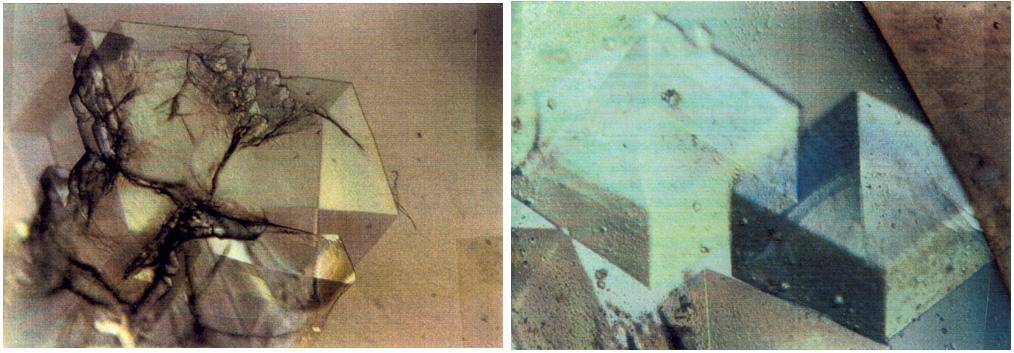
To the crystallographer, this may not be a diamond but it is just as priceless. A Lysozyme crystal grown in orbit looks great under a microscope, but the real test is X-ray crystallography. The colors are caused by polarizing filters. Proteins can form crystals generated by rows and columns of molecules that form up like soldiers on a parade ground. Shining X-rays through a crystal will produce a pattern of dots that can be decoded to reveal the arrangement of the atoms in the molecules making up the crystal. Like the troops in formation, uniformity and order are everything in X-ray crystallography. X-rays have much shorter wavelengths than visible light, so the best looking crystals under the microscope won't necessarily pass muster under the X-rays. In order to have crystals to use for X-ray diffraction studies, crystals need to be fairly large and well ordered. Scientists also need lots of crystals since exposure to air, the process of X-raying them, and other factors destroy them. Growing protein crystals in space has yielded striking results. Lysozyme's structure is well known and it has become a standard in many crystallization studies on Earth and in space.

STS043-03-001 (2-11 Aug 1991) --- Astronaut Shannon W. Lucid, STS-43 mission specialist, is pictured with a sample from the Bio-serve Instrumentation Technology Associates Materials Dispersion Apparatus (BIMDA). BIMDA is designed to obtain data on scientific methods and commercial potential for growing large high quality protein crystals in microgravity. The experimental focus is on both synthetic and natural biological processes that provide the foundation of the assembly of large structures from macromolecules. In addition, cell processes and membrane (cell and artificial) processes are being evaluated. BIMDA experiments are stored and operated on the middeck in a refrigerator/incubator module (R/IM). During this flight, the R/IM maintains a constant internal temperature of 20 degrees Celsius. This experiment also flew on NASA?s STS-37 mission.
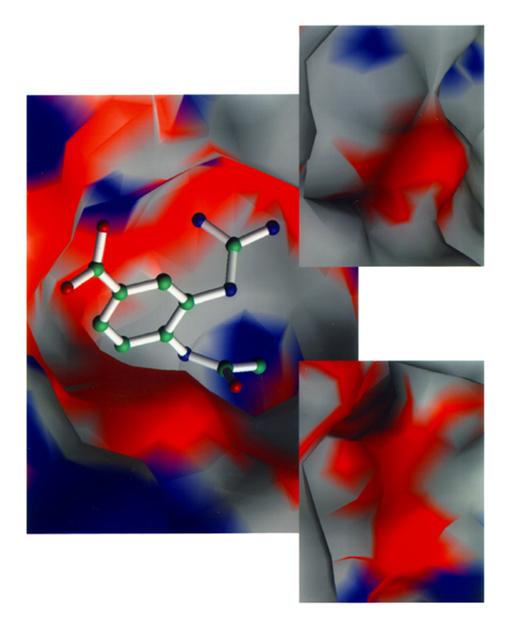
The loss of productivity due to flu is staggering. Costs range as much as $20 billio a year. High mutation rates of the flu virus have hindered development of new drugs or vaccines. The secret lies in a small molecule which is attached to the host cell's surface. Each flu virus, no matter what strain, must remove this small molecule to escape the host cell to spread infection. Using data from space and earth grown crystals, researchers from the Center of Macromolecular Crystallography (CMC) are desining drugs to bind with this protein's active site. This lock and key fit reduces the spread of flu in the body by blocking its escape route. In collaboration with its corporate partner, the CMC has refined drug structure in preparation for clinical trials. Tested and approved relief is expected to reach drugstores by year 2004.