
Scientists at NASA's Marshall Space Flight Center in Huntsville, Alabama, clean equipment and prepare for shipment of the ring sheared drop payload currently set for launch on Northrop Grumman 16 the first week in August, 2021. The payload studies the formation of potentially destructive amyloid fibrils, or protein clusters, like those found in the brain tissue of patients battling neurodegenerative diseases. Such illnesses may damage neurons, the drivers of the human nervous system. Experimentation in microgravity provides the opportunity to study amyloid fibril formation in conditions more analogous to those found in the human body than can be studied in a ground-based laboratory environment.

Scientists at NASA's Marshall Space Flight Center in Huntsville, Alabama, clean equipment and prepare for shipment of the ring sheared drop payload currently set for launch on Northrop Grumman 16 the first week in August, 2021. The payload studies the formation of potentially destructive amyloid fibrils, or protein clusters, like those found in the brain tissue of patients battling neurodegenerative diseases. Such illnesses may damage neurons, the drivers of the human nervous system. Experimentation in microgravity provides the opportunity to study amyloid fibril formation in conditions more analogous to those found in the human body than can be studied in a ground-based laboratory environment.

Scientists at NASA's Marshall Space Flight Center in Huntsville, Alabama, clean equipment and prepare for shipment of the ring sheared drop payload currently set for launch on Northrop Grumman 16 the first week in August, 2021. The payload studies the formation of potentially destructive amyloid fibrils, or protein clusters, like those found in the brain tissue of patients battling neurodegenerative diseases. Such illnesses may damage neurons, the drivers of the human nervous system. Experimentation in microgravity provides the opportunity to study amyloid fibril formation in conditions more analogous to those found in the human body than can be studied in a ground-based laboratory environment.
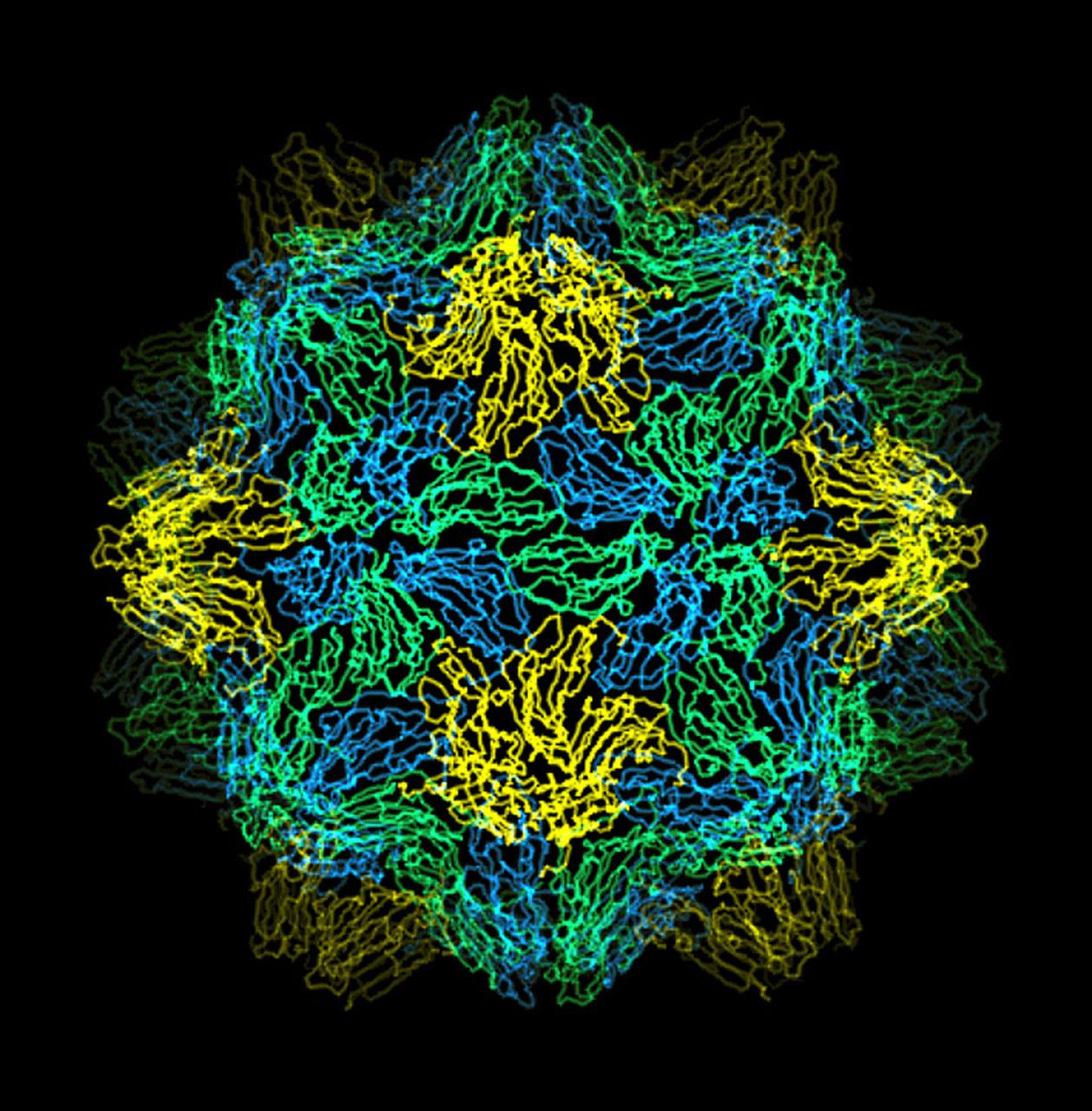
The bumpy exterior of the turnip yellow mosaic virus (TYMV) protein coat, or capsid, was defined in detail by Dr. Alexander McPherson of the University of California, Irvin using protein crystallized in space for analysis on Earth. TYMV is an icosahedral virus constructed from 180 copies of the same protein arranged into 12 clusters of five proteins (pentamers), and 20 clusters of six proteins (hexamers). The final TYMV structure led to the enexpected hypothesis that the virus release its RNA by essentially chemical-mechanical means. Most viruses have farly flat coats, but in TYMV, the fold in each protein, called the jellyroll, is clustered at the points where the protein pentamers and hexamers join. The jellyrolls are almost standing on end, producing a bumpy surface with knobs at all of the pentamers and hexamers. At the inside surface of the pentamers is a void that is not present at the hexamers. The coating had been seen in early studies of TYMV, but McPhereson's atomic structure shows much more detail. The inside surface is strikingly, and unexpectedly, different than the outside. While the pentamers contain a central viod on the inside, the hexameric units contain peptides liked to each other, forming a ring or, more accurately, rings to fill the voild. Credit: Dr. Alexander McPherson, University of California, Irvine.

The bumpy exterior of the turnip yellow mosaic virus (TYMV) protein coat, or capsid, was defined in detail by Dr. Alexander McPherson of the University of California, Irvin using proteins crystallized in space for analysis on Earth. TYMV is an icosahedral virus constructed from 180 copies of the same protein arranged into 12 clusters of five proteins (pentamers), and 20 clusters of six proteins (hexamers). The final TYMV structure led to the unexpected hypothesis that the virus releases its RNA by essentially chemical-mechanical means. Most viruses have fairly flat coats, but in TYNV, the fold in each protein, called the jellyroll, is clustered at the points where the protein pentamers and hexamers join. The jellyrolls are almost standing on end, producing a bumpy surface with knobs at all of the pentamers and hexamers. At the inside surface of the pentamers is a void that is not present at the hexamers. The coating had been seen in early stuties of TYMV, but McPherson's atomic structure shows much more detail. The inside surface is strikingly, and unexpectedly, different than the outside. While the pentamers contain a central void on the inside, the hexameric units contain peptides linked to each other, forming a ring or, more accurately, rings to fill the void. Credit: Dr. Alexander McPherson, University of California, Irvine

iss067e378812 (Sept. 21, 2022) --- Expedition 67 Flight Engineer and ESA (European Space Agency) astronaut Samantha Cristoforetti works inside the Microgravity Science Glovebox removing hardware that supported the Ring Sheared Drop experiment. The fluid physics study observes the formation of destructive protein clusters that may be responsible for neurodegenerative diseases such as Alzheimer’s.
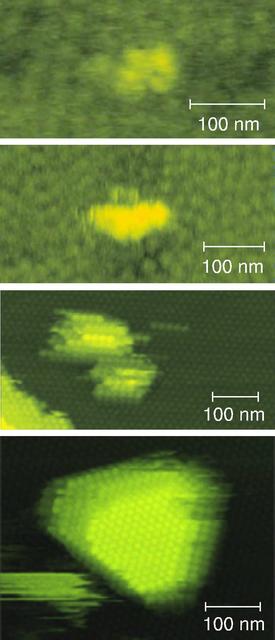
Watching molecules of the iron-storing protein apoferritin come together to form a nucleus reveals some interesting behavior. In this series of images, researchers observed clusters of four molecules at the corners of a diamond shape (top). As more molecules attach to the cluster, they arrange themselves into rods (second from top), and a raft-like configuration of molecules forms the critical nucleus (third from top), suggesting that crystal growth is much slower than it could be were the molecules arranged in a more compact formation. In the final image, a crystallite consisting of three layers containing approximately 60 to 70 molecules each is formed. Atomic force microscopy made visualizing the process of nucleation possible for the first time. The principal investigator is Peter Vekilov, of the University of Alabama in Huntsville. Vekilov's team at UAH studies protein solutions as they change phases from liquids to crystalline solids. They want to know if the molecules in the solution interact with one another, and if so, how, from the perspectives of thermodynamics and kinetics. They want to understand which forces -- electrical, electrostatic, hydrodynamic, or other kinds of forces -- are responsible for the interactions. They also study nucleation, the begirning stage of crystallization. This process is important to understand because it sets the stage for crystal growth in all kinds of solutions and liquid melts that are important in such diverse fields as agriculture, medicine, and the fabrication of metal components. Nucleation can determine the rate of crystal growth, the number of crystals that will be formed, and the quality and size of the crystals.