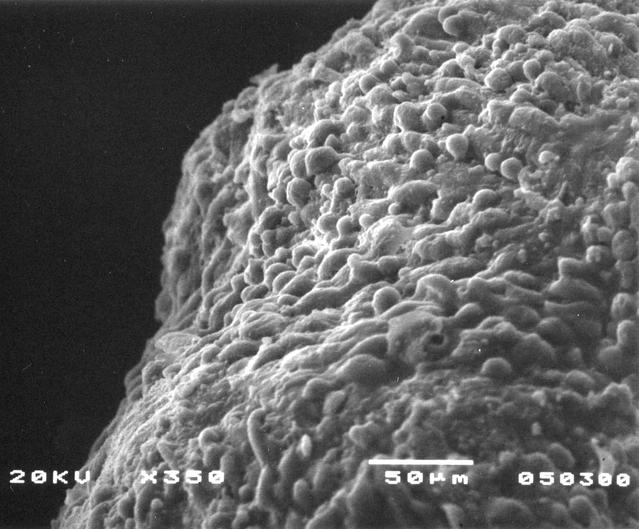
This prostate cancer construct was grown during NASA-sponsored bioreactor studies on Earth. Cells are attached to a biodegradable plastic lattice that gives them a head start in growth. Prostate tumor cells are to be grown in a NASA-sponsored Bioreactor experiment aboard the STS-107 Research-1 mission in 2002. Dr. Leland Chung of the University of Virginia is the principal investigator. The NASA Bioreactor provides a low turbulence culture environment which promotes the formation of large, three-dimensional cell clusters. Due to their high level of cellular organization and specialization, samples constructed in the bioreactor more closely resemble the original tumor or tissue found in the body. The Bioreactor is rotated to provide gentle mixing of fresh and spent nutrient without inducing shear forces that would damage the cells. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators. Credit: NASA and the University of Virginia.
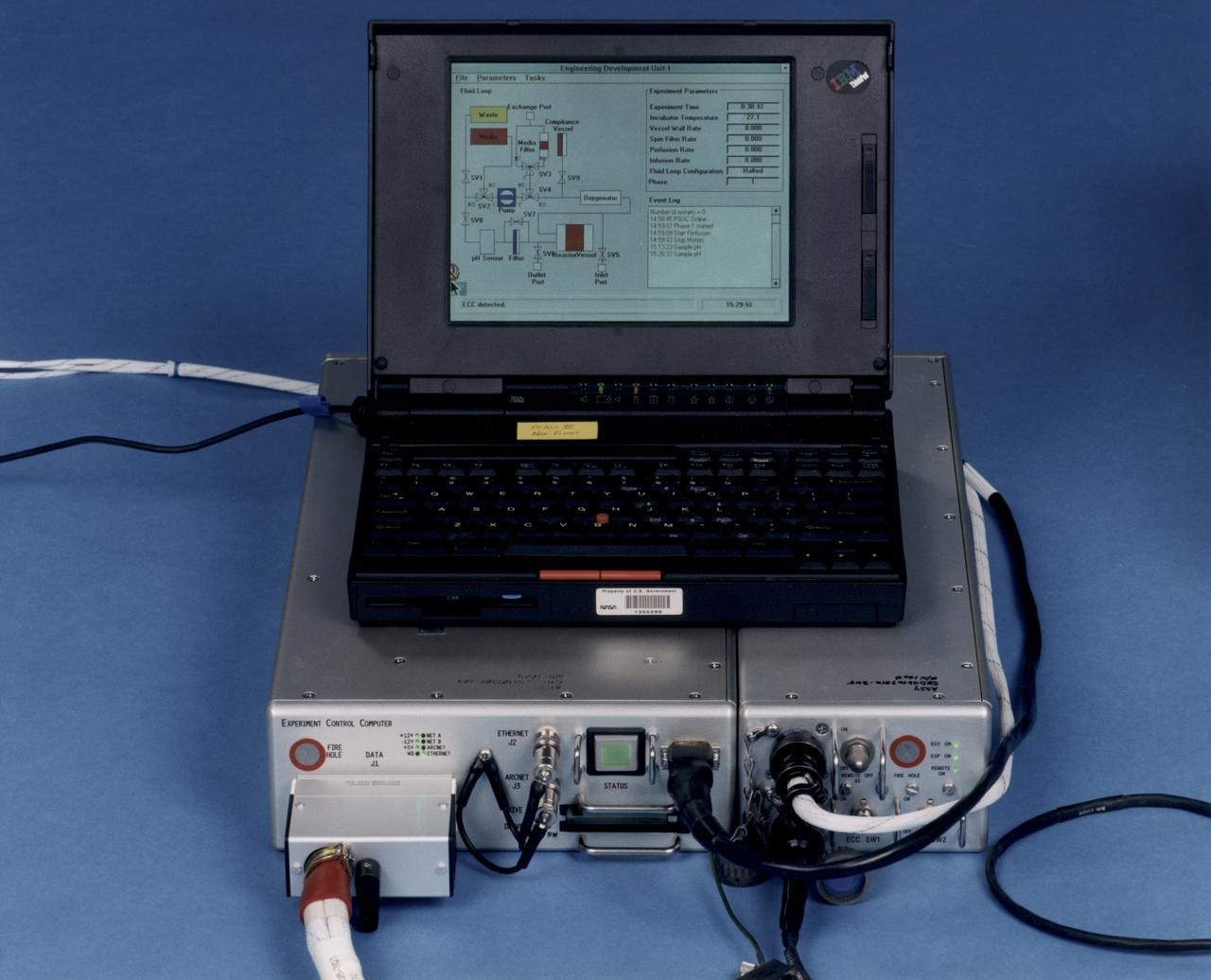
Laptop computer sits atop the Experiment Control Computer for a NASA Bioreactor. The flight crew can change operating conditions in the Bioreactor by using the graphical interface on the laptop. The NASA Bioreactor provides a low turbulence culture environment which promotes the formation of large, three-dimensional cell clusters. The Bioreactor is rotated to provide gentle mixing of fresh and spent nutrient without inducing shear forces that would damage the cells. Due to their high level of cellular organization and specialization, samples constructed in the bioreactor more closely resemble the original tumor or tissue found in the body. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators.
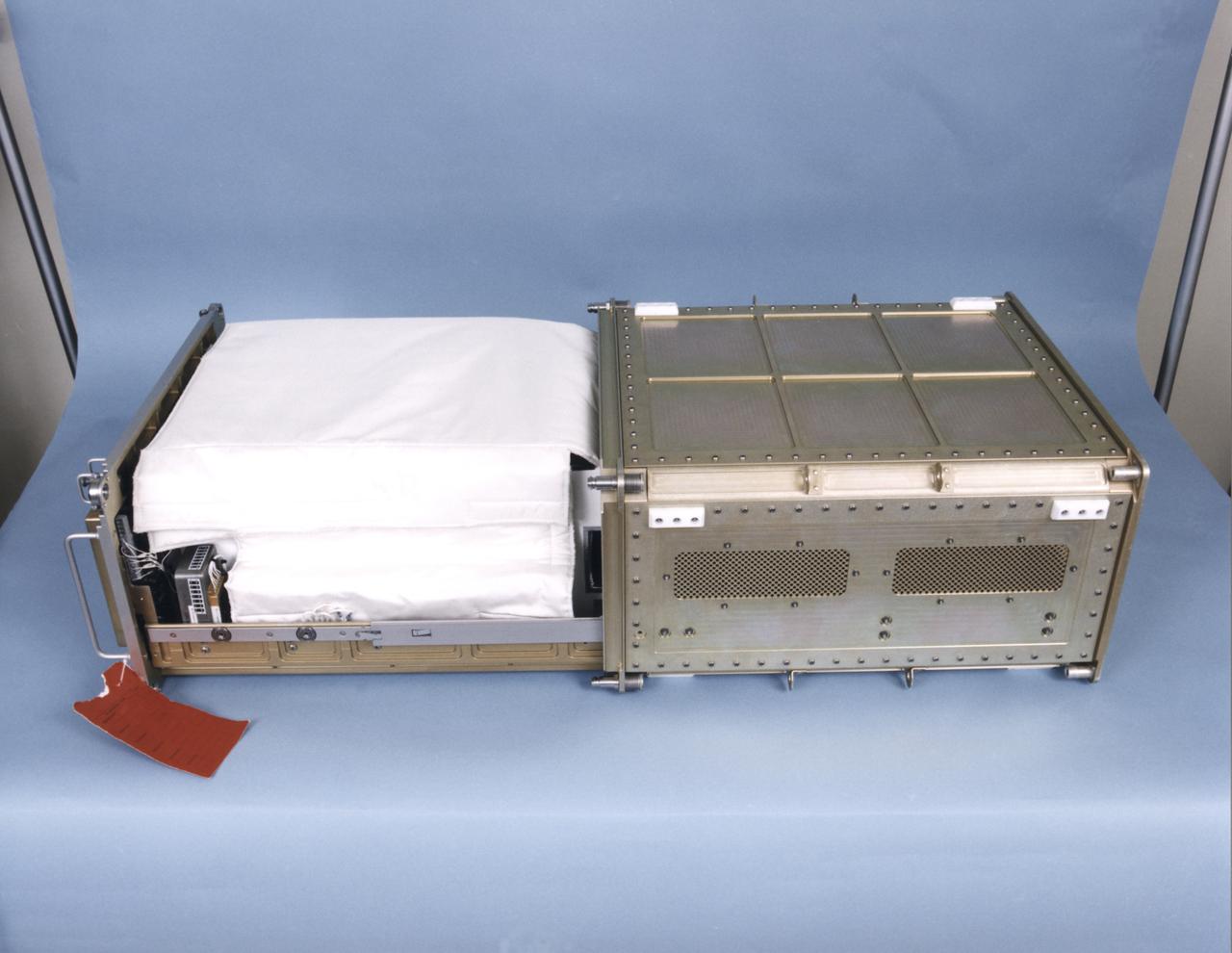
Biotechnology Refrigerator that preserves samples for use in (or after culturing in) the NASA Bioreactor. The unit is shown extracted from a middeck locker shell. The NASA Bioreactor provides a low turbulence culture environment which promotes the formation of large, three-dimensional cell clusters. The Bioreactor is rotated to provide gentle mixing of fresh and spent nutrient without inducing shear forces that would damage the cells. Due to their high level of cellular organization and specialization, samples constructed in the bioreactor more closely resemble the original tumor or tissue found in the body. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators.
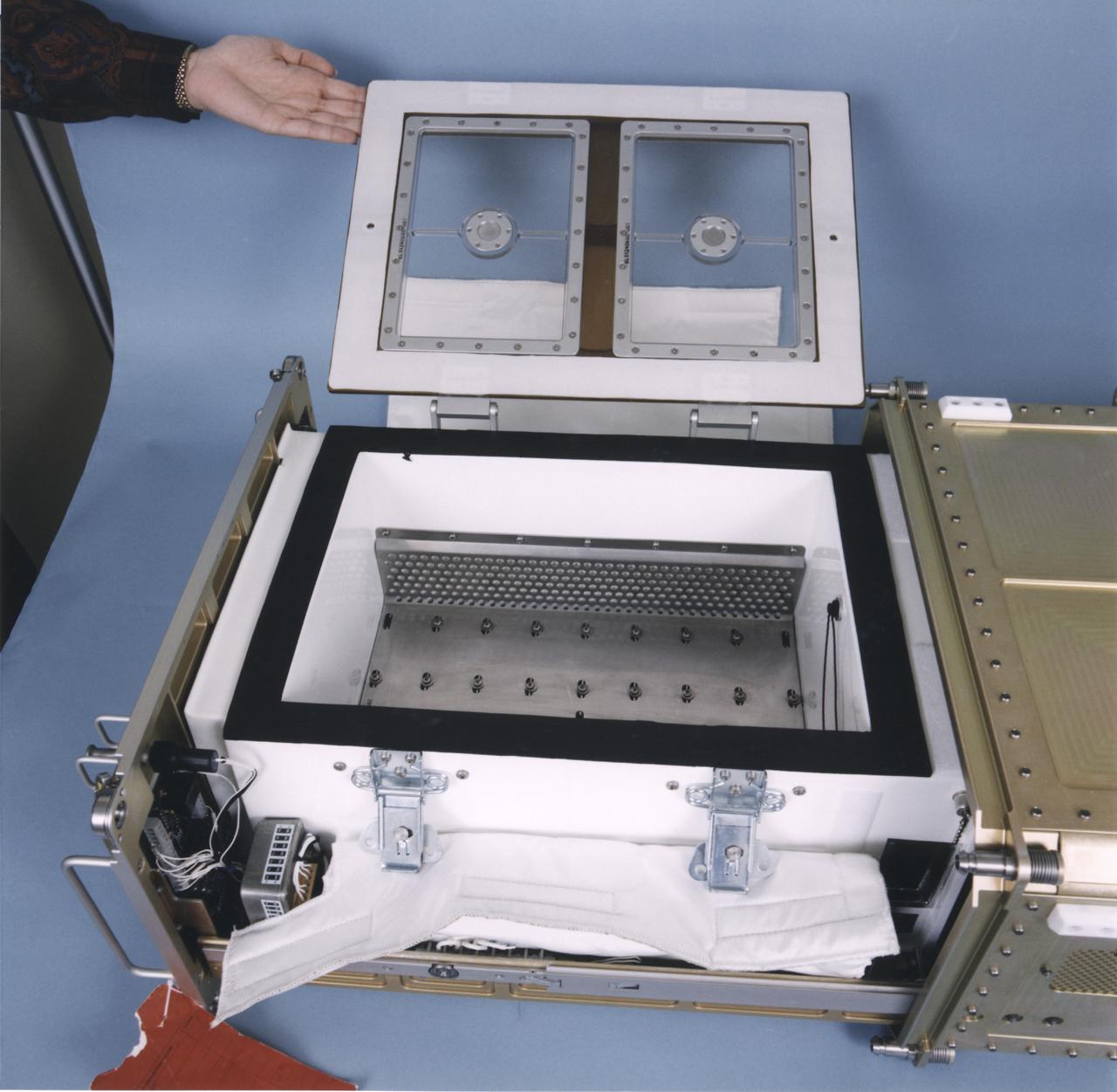
Interior of a Biotechnology Refrigerator that preserves samples for use in (or after culturing in) the NASA Bioreactor. The unit is shown extracted from a middeck locker shell. The NASA Bioreactor provides a low turbulence culture environment which promotes the formation of large, three-dimensional cell clusters. The Bioreactor is rotated to provide gentle mixing of fresh and spent nutrient without inducing shear forces that would damage the cells. Due to their high level of cellular organization and specialization, samples constructed in the bioreactor more closely resemble the original tumor or tissue found in the body. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators.
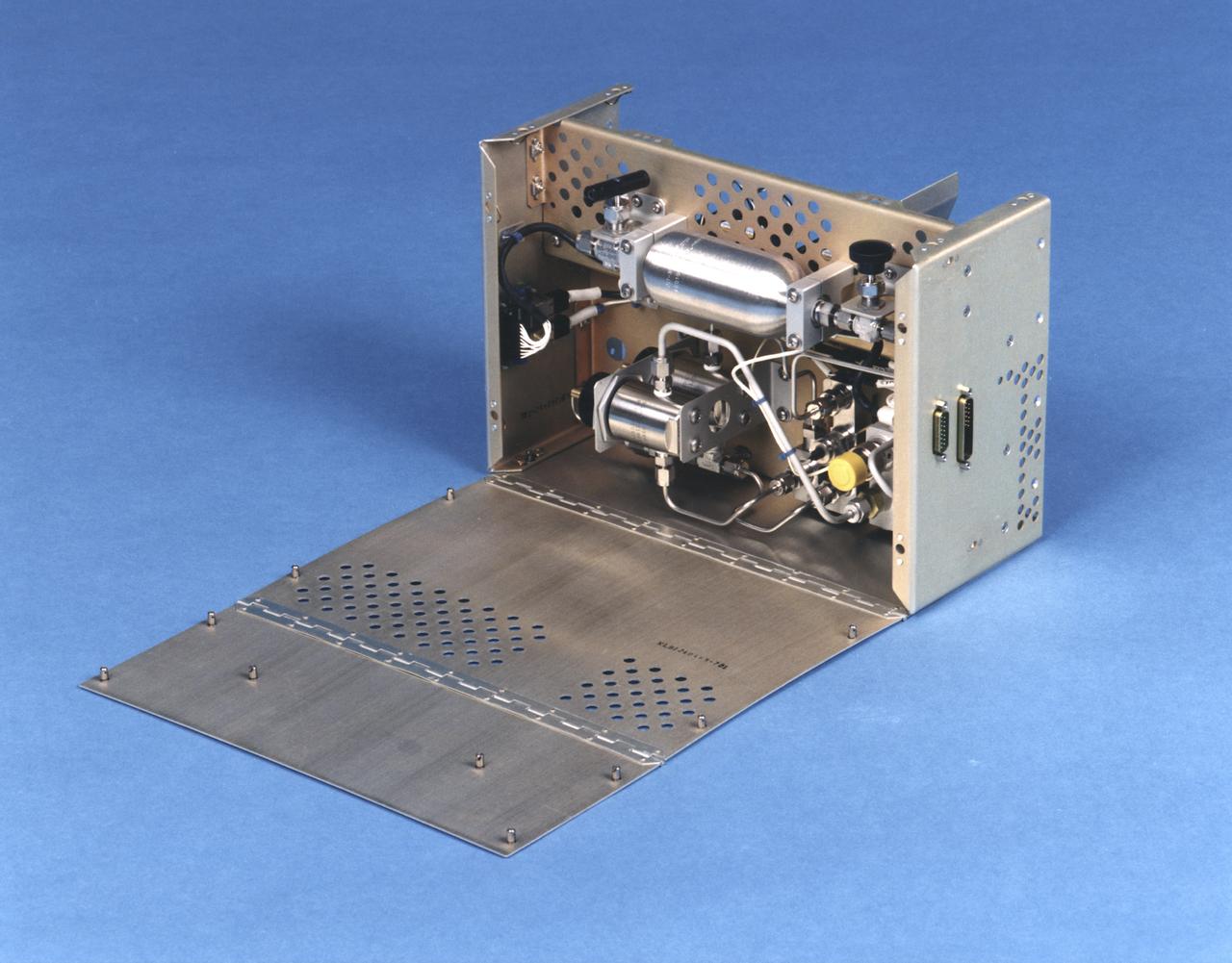
Interior view of the gas supply for the NASA Bioreactor. The NASA Bioreactor provides a low turbulence culture environment which promotes the formation of large, three-dimensional cell clusters. The Bioreactor is rotated to provide gentle mixing of fresh and spent nutrient without inducing shear forces that would damage the cells. Due to their high level of cellular organization and specialization, samples constructed in the bioreactor more closely resemble the original tumor or tissue found in the body. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators.
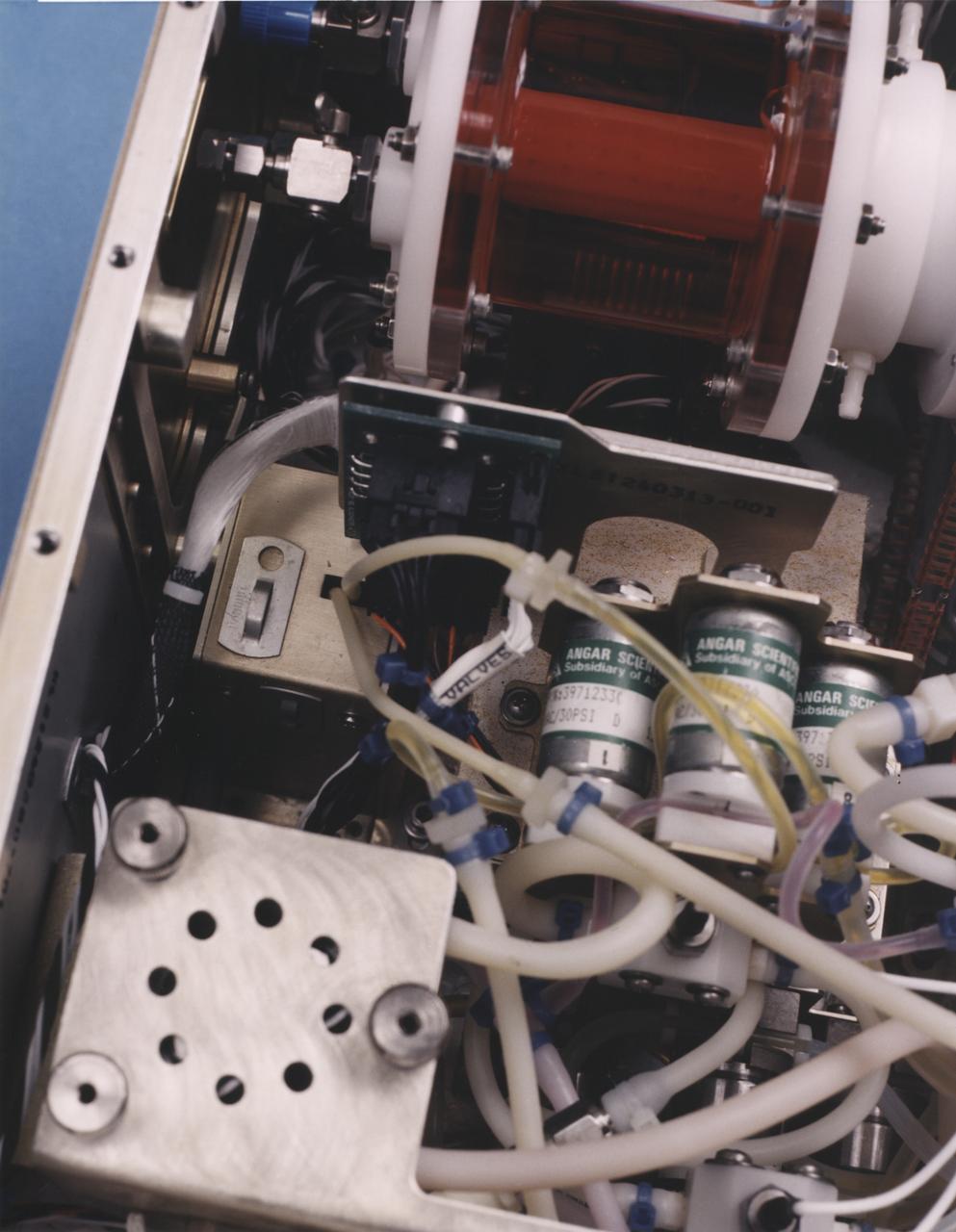
Close-up view of the interior of a NASA Bioreactor shows the plastic plumbing and valves (cylinders at right center) to control fluid flow. The rotating wall vessel is at top center. The NASA Bioreactor provides a low turbulence culture environment which promotes the formation of large, three-dimensional cell clusters. The Bioreactor is rotated to provide gentle mixing of fresh and spent nutrient without inducing shear forces that would damage the cells. Due to their high level of cellular organization and specialization, samples constructed in the bioreactor more closely resemble the original tumor or tissue found in the body. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators.
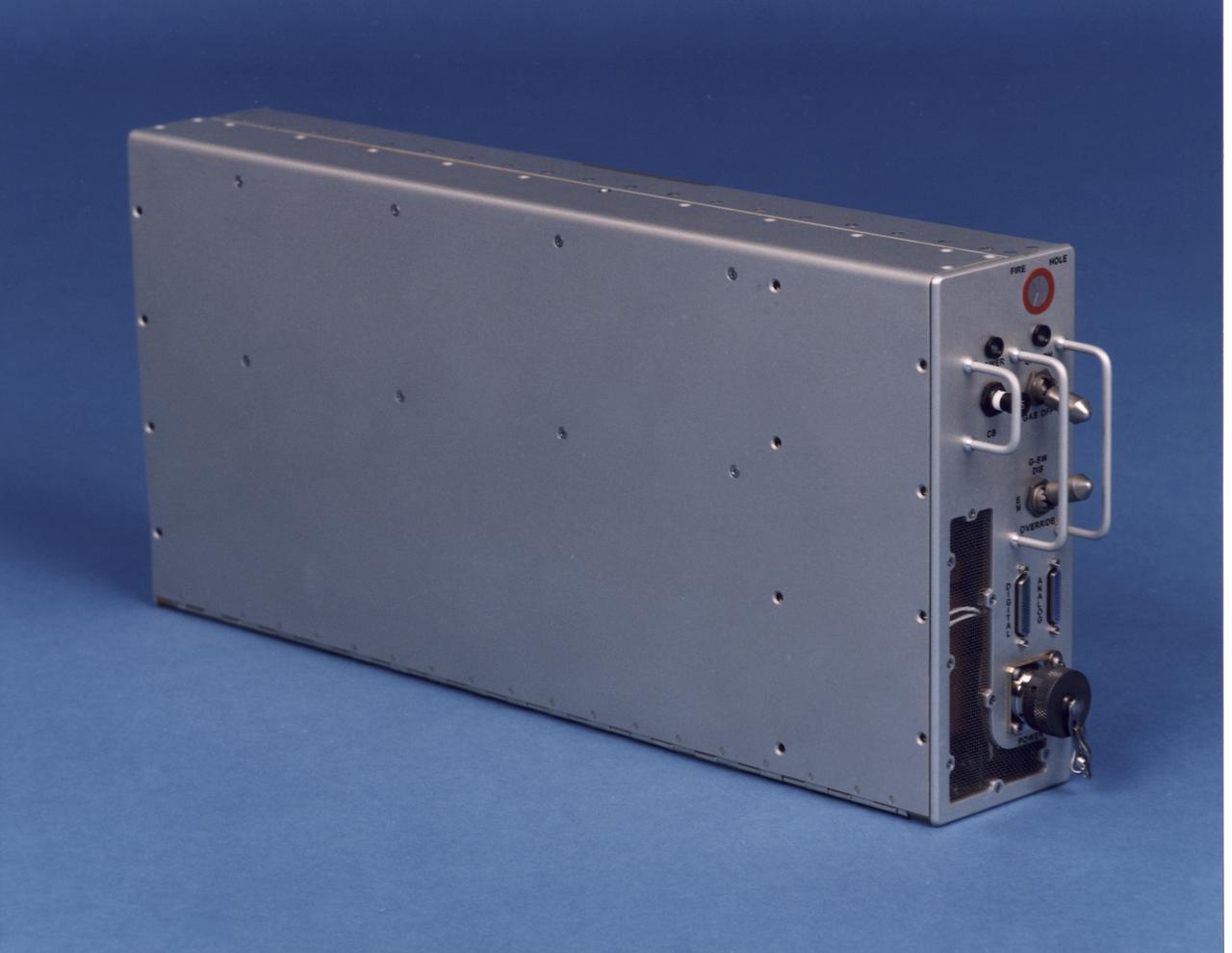
Electronics control module for the NASA Bioreactor. The NASA Bioreactor provides a low turbulence culture environment which promotes the formation of large, three-dimensional cell clusters. The Bioreactor is rotated to provide gentle mixing of fresh and spent nutrient without inducing shear forces that would damage the cells. Due to their high level of cellular organization and specialization, samples constructed in the bioreactor more closely resemble the original tumor or tissue found in the body. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators.
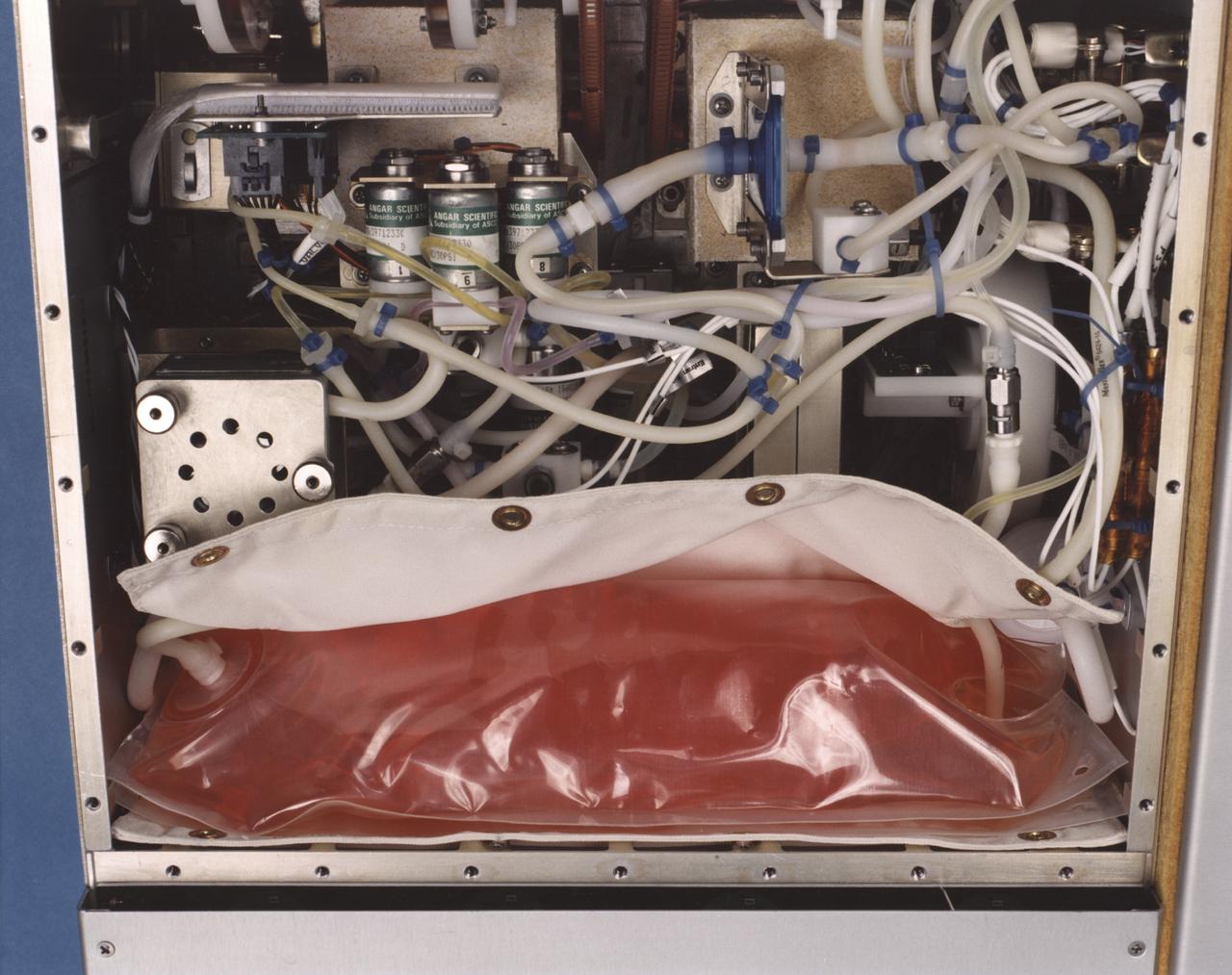
Close-up view of the interior of a NASA Bioreactor shows the plastic plumbing and valves (cylinders at center) to control fluid flow. A fresh nutrient bag is installed at top; a flattened waste bag behind it will fill as the nutrients are consumed during the course of operation. The drive chain and gears for the rotating wall vessel are visible at bottom center center. The NASA Bioreactor provides a low turbulence culture environment which promotes the formation of large, three-dimensional cell clusters. The Bioreactor is rotated to provide gentle mixing of fresh and spent nutrient without inducing shear forces that would damage the cells. Due to their high level of cellular organization and specialization, samples constructed in the bioreactor more closely resemble the original tumor or tissue found in the body. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators.
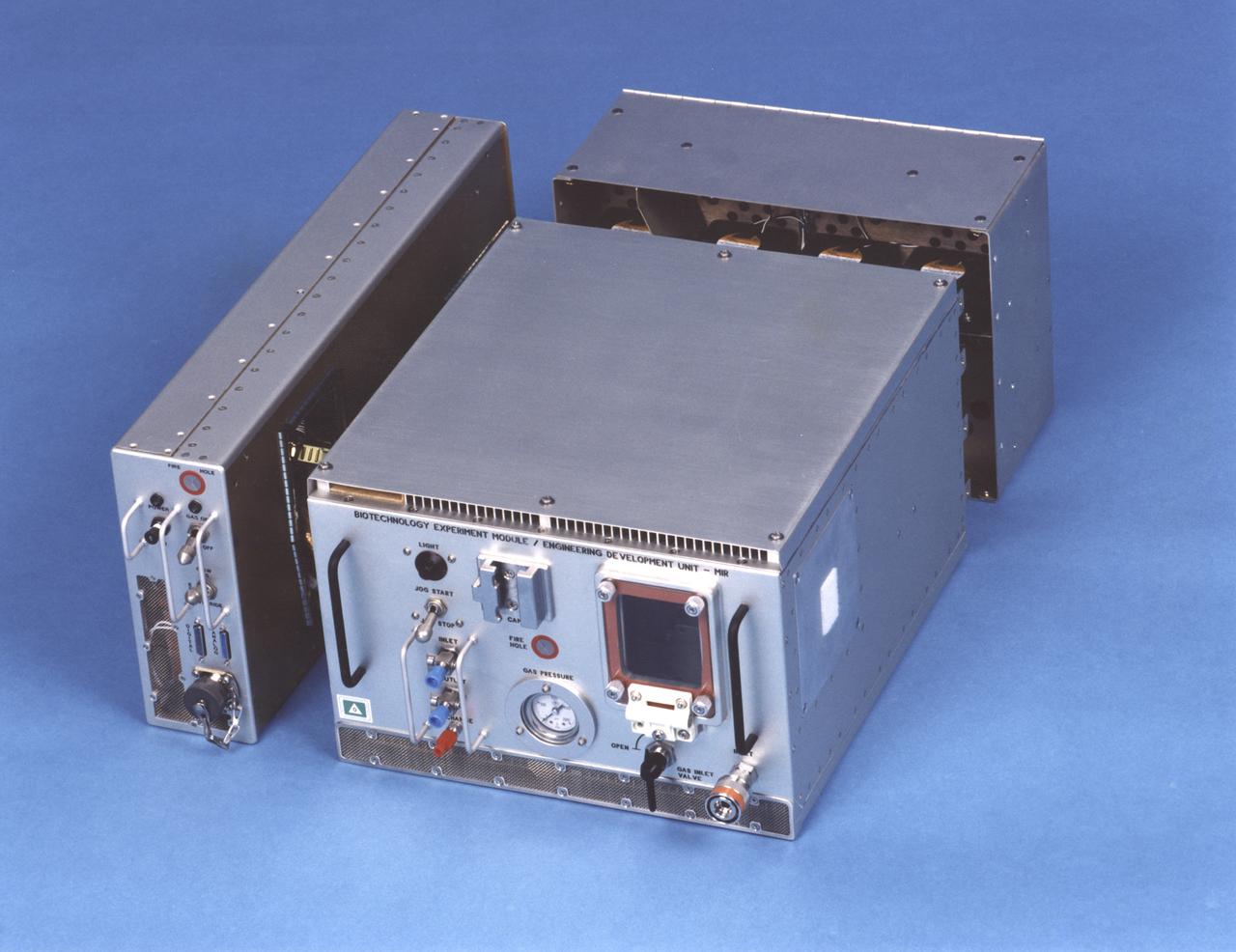
Exterior view of the NASA Bioreactor Engineering Development Unit flown on Mir. The rotating wall vessel is behind the window on the face of the large module. Control electronics are in the module at left; gas supply and cooling fans are in the module at back. The NASA Bioreactor provides a low turbulence culture environment which promotes the formation of large, three-dimensional cell clusters. The Bioreactor is rotated to provide gentle mixing of fresh and spent nutrient without inducing shear forces that would damage the cells. Due to their high level of cellular organization and specialization, samples constructed in the bioreactor more closely resemble the original tumor or tissue found in the body. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators.
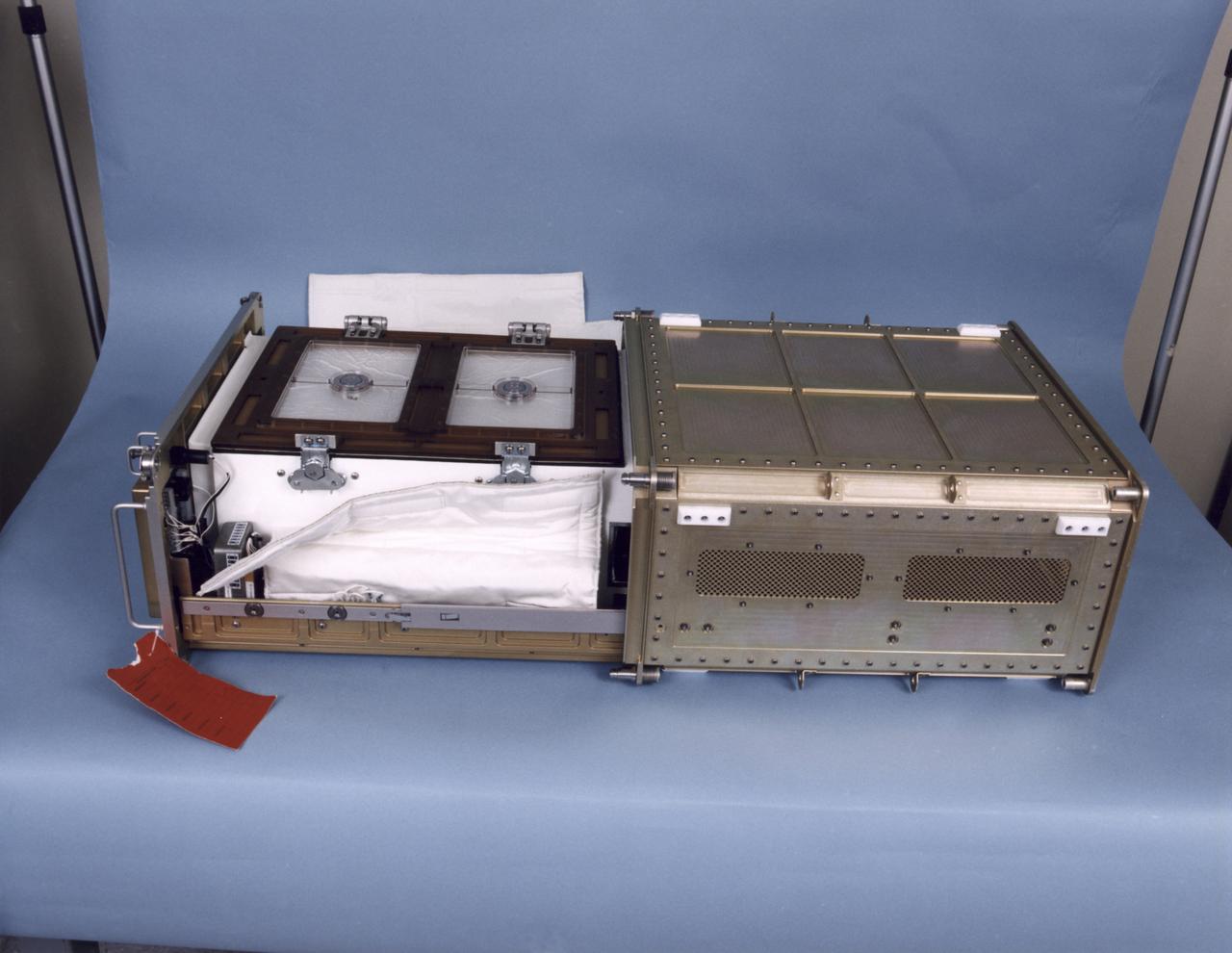
Biotechnology Refrigerator that preserves samples for use in (or after culturing in) the NASA Bioreactor. The unit is shown extracted from a middeck locker shell and with thermal blankets partially removed. The NASA Bioreactor provides a low turbulence culture environment which promotes the formation of large, three-dimensional cell clusters. The Bioreactor is rotated to provide gentle mixing of fresh and spent nutrient without inducing shear forces that would damage the cells. Due to their high level of cellular organization and specialization, samples constructed in the bioreactor more closely resemble the original tumor or tissue found in the body. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators.
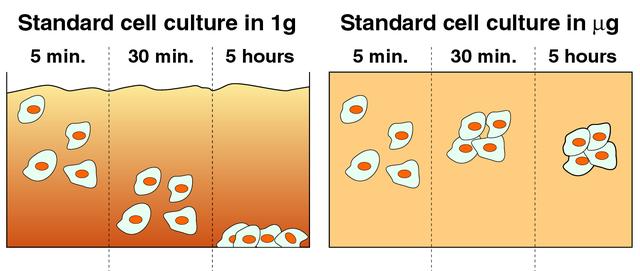
Cells cultured on Earth (left) typically settle quickly on the bottom of culture vessels due to gravity. In microgravity (right), cells remain suspended and aggregate to form three-dimensional tissue. The NASA Bioreactor provides a low turbulence culture environment which promotes the formation of large, three-dimensional cell clusters. The Bioreactor is rotated to provide gentle mixing of fresh and spent nutrient without inducing shear forces that would damage the cells. Due to their high level of cellular organization and specialization, samples constructed in the bioreactor more closely resemble the original tumor or tissue found in the body. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators.
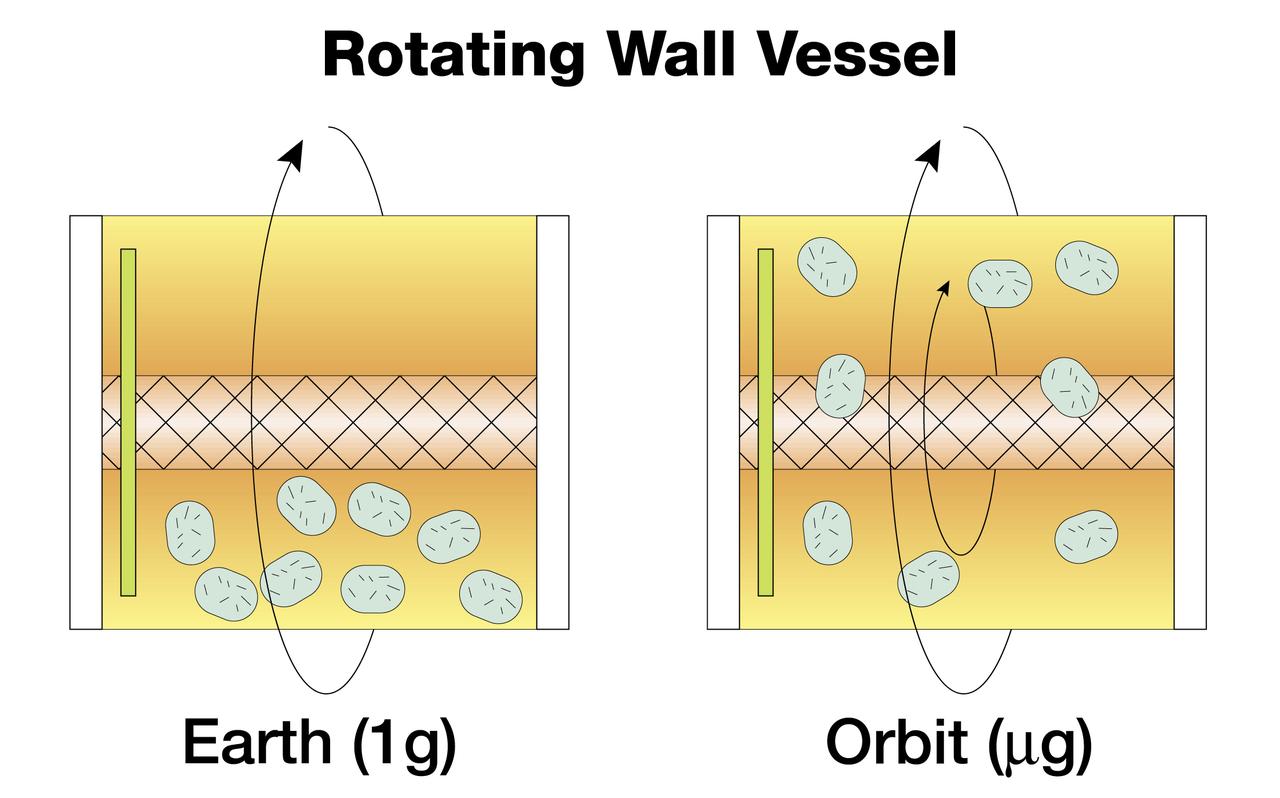
The NASA Bioreactor provides a low turbulence culture environment which promotes the formation of large, three-dimensional cell clusters. Due to their high level of cellular organization and specialization, samples constructed in the bioreactor more closely resemble the original tumor or tissue found in the body. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators. Cell constructs grown in a rotating bioreactor on Earth (left) eventually become too large to stay suspended in the nutrient media. In the microgravity of orbit, the cells stay suspended. Rotation then is needed for gentle stirring to replenish the media around the cells.

Astronaut John Blaha replaces an exhausted media bag and filled waste bag with fresh bags to continue a bioreactor experiment aboard space station Mir in 1996. NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators. This image is from a video downlink. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC).

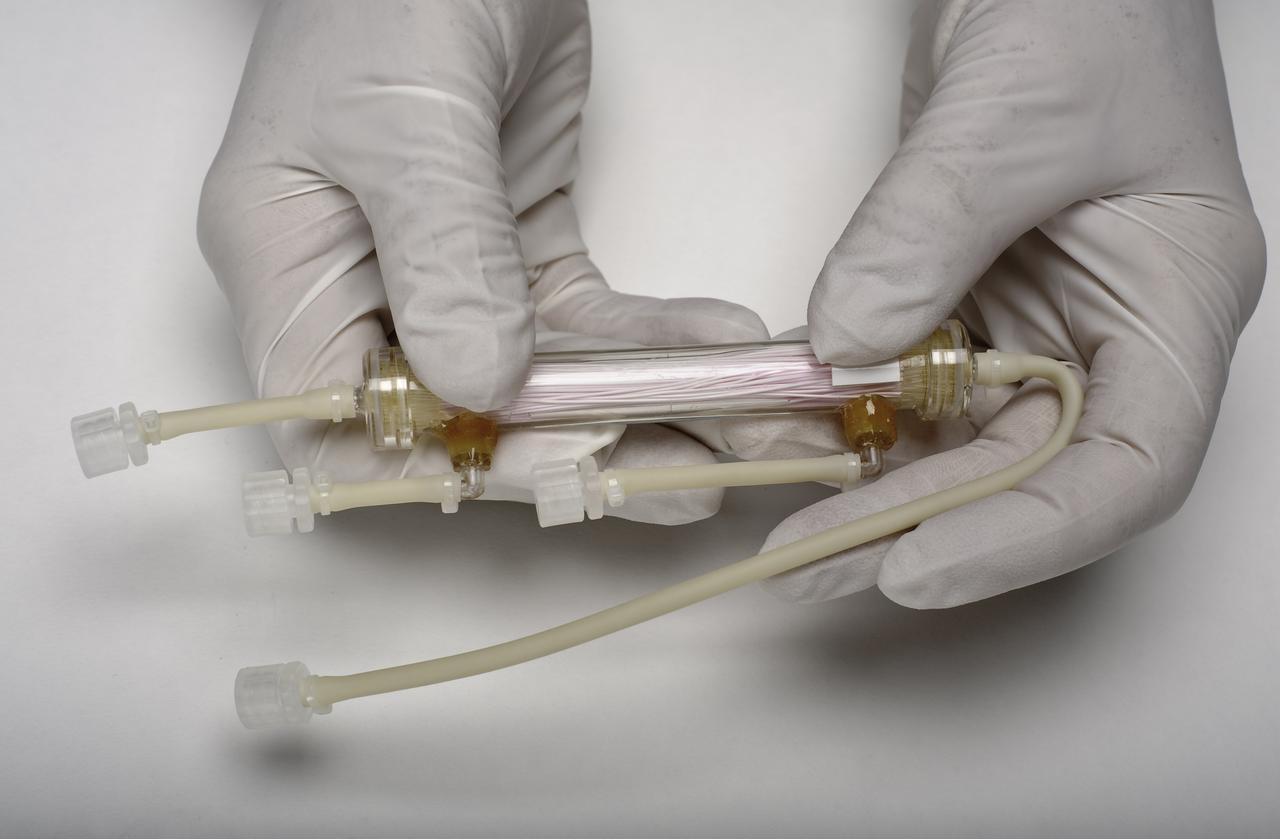
The heart of the bioreactor is the rotating wall vessel, shown without its support equipment. Volume is about 125 mL. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators.
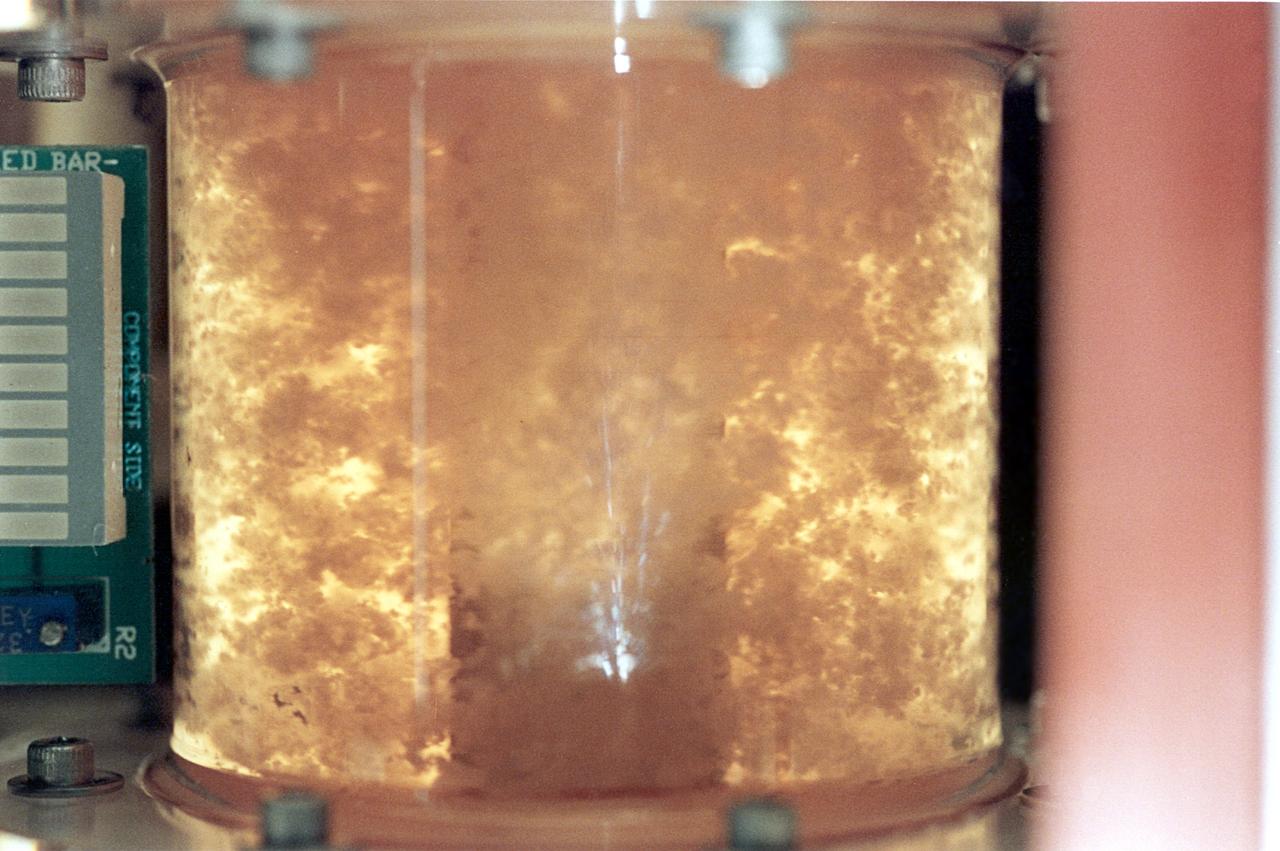
For 5 days on the STS-70 mission, a bioreactor cultivated human colon cancer cells, which grew to 30 times the volume of control specimens grown on Earth. This significant result was reproduced on STS-85 which grew mature structures that more closely match what are found in tumors in humans. Shown here, clusters of cells slowly spin inside a bioreactor. On Earth, the cells continually fall through the buffer medium and never hit bottom. In space, they are naturally suspended. Rotation ensures gentle stirring so waste is removed and fresh nutrient and oxygen are supplied. The NASA Bioreactor provides a low turbulence culture environment which promotes the formation of large, three-dimensional cell clusters. Due to their high level of cellular organization and specialization, samples constructed in the bioreactor more closely resemble the original tumor or tissue found in the body. The Bioreactor is rotated to provide gentle mixing of fresh and spent nutrient without inducing shear forces that would damage the cells. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators.

Lisa Freed and Gordana Vunjak-Novakovic, both of the Massachusetts Institute of Technology (MIT), have taken the first steps toward engineering heart muscle tissue that could one day be used to patch damaged human hearts. Cells isolated from very young animals are attached to a three-dimensional polymer scaffold, then placed in a NASA bioreactor. The cells do not divide, but after about a week start to cornect to form a functional piece of tissue. Here, a transmission electron micrograph of engineered tissue shows a number of important landmarks present in functional heart tissue: (A) well-organized myofilaments (Mfl), z-lines (Z), and abundant glycogen granules (Gly); and (D) intercalcated disc (ID) and desmosomes (DES). The NASA Bioreactor provides a low turbulence culture environment which promotes the formation of large, three-dimensional cell clusters. The Bioreactor is rotated to provide gentle mixing of fresh and spent nutrient without inducing shear forces that would damage the cells. Due to their high level of cellular organization and specialization, samples constructed in the bioreactor more closely resemble the original tumor or tissue found in the body. NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). Credit: MIT
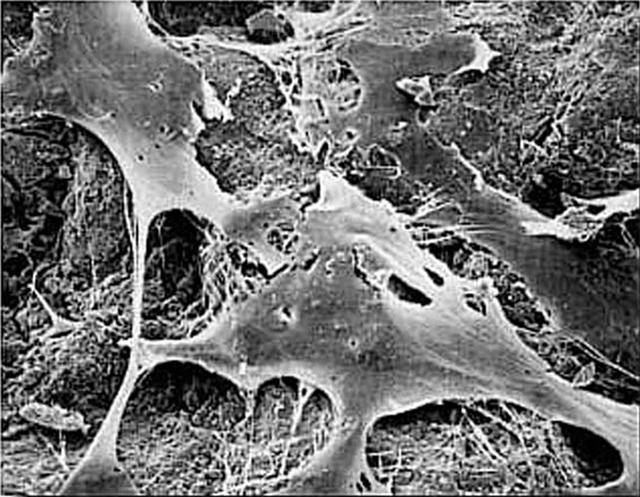
Paul Ducheyne, a principal investigator in the microgravity materials science program and head of the University of Pernsylvania's Center for Bioactive Materials and Tissue Engineering, is leading the trio as they use simulated microgravity to determine the optimal characteristics of tiny glass particles for growing bone tissue. The result could make possible a much broader range of synthetic bone-grafting applications. Even in normal gravity, bioactive glass particles enhance bone growth in laboratory tests with flat tissue cultures. Ducheyne and his team believe that using the bioactive microcarriers in a rotating bioreactor in microgravity will produce improved, three-dimensional tissue cultures. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators. Credit: NASA and University of Pennsylvania Center for Bioactive Materials and Tissue Engineering.
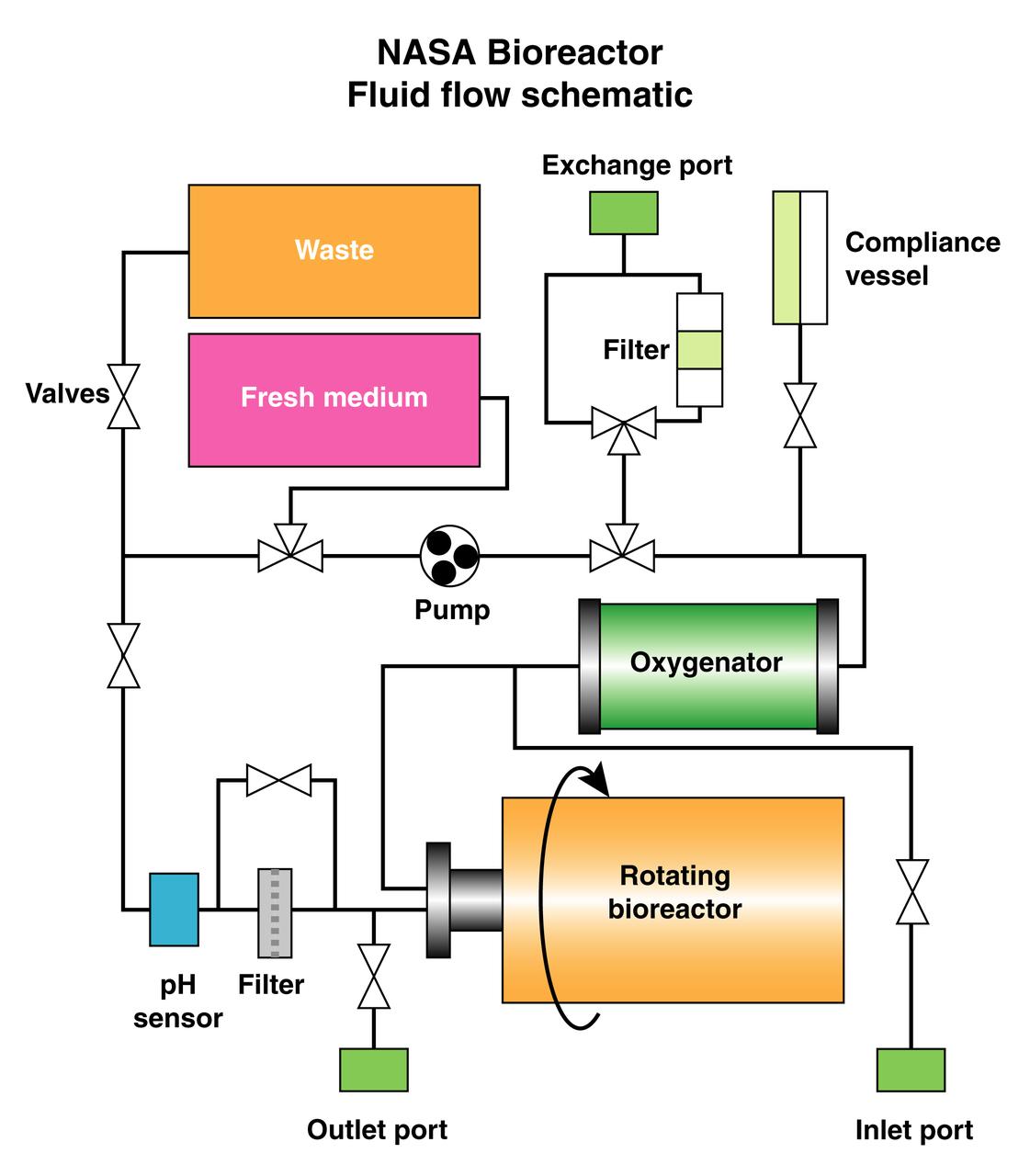
The schematic depicts the major elements and flow patterns inside the NASA Bioreactor system. Waste and fresh medium are contained in plastic bags placed side-by-side so the waste bag fills as the fresh medium bag is depleted. The compliance vessel contains a bladder to accommodate pressure transients that might damage the system. A peristolic pump moves fluid by squeezing the plastic tubing, thus avoiding potential contamination. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators.

Biotechnology Refrigerator (BTR) holds fixed tissue culture bags at 4 degrees C to preserve them for return to Earth and postflight analysis. The cultures are used in research with the NASA Bioreactor cell science program. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC).
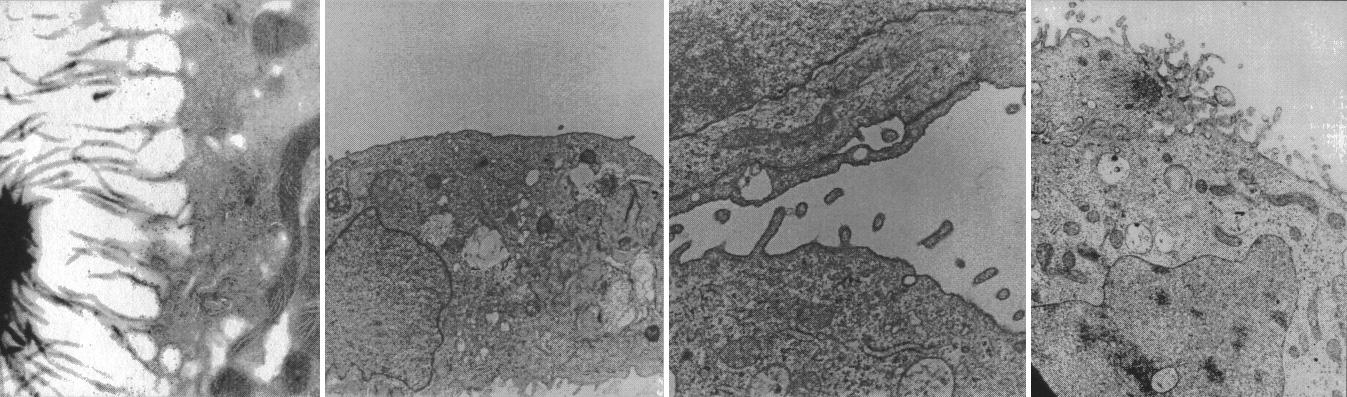
Cells from kidneys lose some of their special features in conventional culture but form spheres replete with specialized cell microvilli (hair) and synthesize hormones that may be clinically useful. Ground-based research studies have demonstrated that both normal and neoplastic cells and tissues recreate many of the characteristics in the NASA bioreactor that they display in vivo. Proximal kidney tubule cells that normally have rich apically oriented microvilli with intercellular clefts in the kidney do not form any of these structures in conventional two-dimensional monolayer culture. However, when normal proximal renal tubule cells are cultured in three-dimensions in the bioreactor, both the microvilli and the intercellular clefts form. This is important because, when the morphology is recreated, the function is more likely also to be rejuvenated. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC).

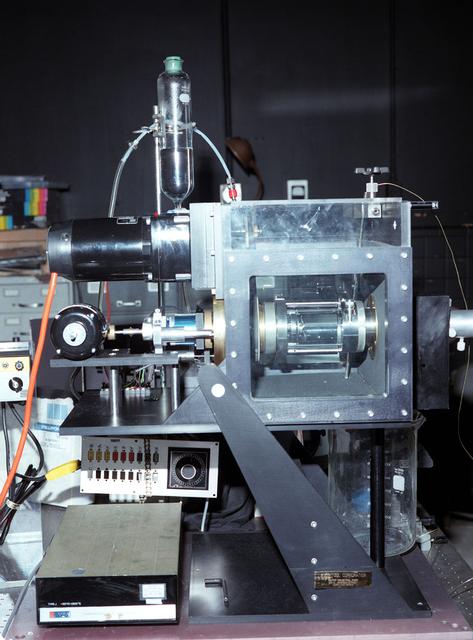
Bioreactor Demonstration System (BDS) comprises an electronics module, a gas supply module, and the incubator module housing the rotating wall vessel and its support systems. Nutrient media are pumped through an oxygenator and the culture vessel. The shell rotates at 0.5 rpm while the irner filter typically rotates at 11.5 rpm to produce a gentle flow that ensures removal of waste products as fresh media are infused. Periodically, some spent media are pumped into a waste bag and replaced by fresh media. When the waste bag is filled, an astronaut drains the waste bag and refills the supply bag through ports on the face of the incubator. Pinch valves and a perfusion pump ensure that no media are exposed to moving parts. An Experiment Control Computer controls the Bioreactor, records conditions, and alerts the crew when problems occur. The crew operates the system through a laptop computer displaying graphics designed for easy crew training and operation. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators. See No. 0101824 for a version with labels, and No. 0103180 for an operational schematic.
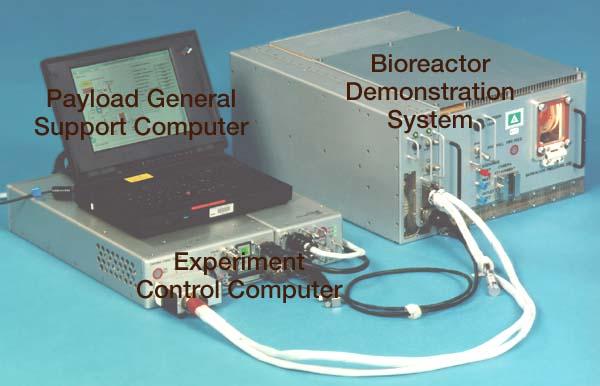
Bioreactor Demonstration System (BDS) comprises an electronics module, a gas supply module, and the incubator module housing the rotating wall vessel and its support systems. Nutrient media are pumped through an oxygenator and the culture vessel. The shell rotates at 0.5 rpm while the irner filter typically rotates at 11.5 rpm to produce a gentle flow that ensures removal of waste products as fresh media are infused. Periodically, some spent media are pumped into a waste bag and replaced by fresh media. When the waste bag is filled, an astronaut drains the waste bag and refills the supply bag through ports on the face of the incubator. Pinch valves and a perfusion pump ensure that no media are exposed to moving parts. An Experiment Control Computer controls the Bioreactor, records conditions, and alerts the crew when problems occur. The crew operates the system through a laptop computer displaying graphics designed for easy crew training and operation. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators. See No. 0101825 for a version with major elements labeled, and No. 0103180 for an operational schematic. 0101816

Bioreactor Demonstration System (BDS) comprises an electronics module, a gas supply module, and the incubator module housing the rotating wall vessel and its support systems. Nutrient media are pumped through an oxygenator and the culture vessel. The shell rotates at 0.5 rpm while the irner filter typically rotates at 11.5 rpm to produce a gentle flow that ensures removal of waste products as fresh media are infused. Periodically, some spent media are pumped into a waste bag and replaced by fresh media. When the waste bag is filled, an astronaut drains the waste bag and refills the supply bag through ports on the face of the incubator. Pinch valves and a perfusion pump ensure that no media are exposed to moving parts. An Experiment Control Computer controls the Bioreactor, records conditions, and alerts the crew when problems occur. The crew operates the system through a laptop computer displaying graphics designed for easy crew training and operation. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators. See No. 0101816 for a version without labels, and No. 0103180 for an operational schematic.

Biotechnology Specimen Temperature Controller (BSTC) will cultivate cells until their turn in the bioreactor; it can also be used in culturing experiments that do not require the bioreactor. The BSTC comprises four incubation/refrigeration chambers individually set at 4 to 50 deg. C (near-freezing to above body temperature). Each chamber holds three rugged tissue chamber modules (12 total), clear Teflon bags holding 30 ml of growth media, all positioned by a metal frame. Every 7 to 21 days (depending on growth rates), an astronaut uses a shrouded syringe and the bags' needleless injection ports to transfer a few cells to a fresh media bag, and to introduce a fixative so that the cells may be studied after flight. The design also lets the crew sample the media to measure glucose, gas, and pH levels, and to inspect cells with a microscope. The controller is monitored by the flight crew through a 23-cm (9-inch) color computer display on the face of the BSTC. This view shows the BTSC with the front panel open. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators.
Bioreactor Demonstration System (BDS) comprises an electronics module, a gas supply module, and the incubator module housing the rotating wall vessel and its support systems. Nutrient media are pumped through an oxygenator and the culture vessel. The shell rotates at 0.5 rpm while the irner filter typically rotates at 11.5 rpm to produce a gentle flow that ensures removal of waste products as fresh media are infused. Periodically, some spent media are pumped into a waste bag and replaced by fresh media. When the waste bag is filled, an astronaut drains the waste bag and refills the supply bag through ports on the face of the incubator. Pinch valves and a perfusion pump ensure that no media are exposed to moving parts. An Experiment Control Computer controls the Bioreactor, records conditions, and alerts the crew when problems occur. The crew operates the system through a laptop computer displaying graphics designed for easy crew training and operation. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators. See No. 0101823 for a version without labels, and No. 0103180 for an operational schematic.
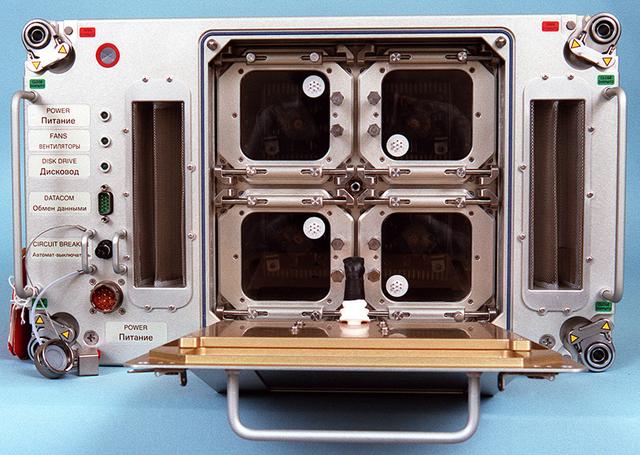
Biotechnology Specimen Temperature Controller (BSTC) will cultivate cells until their turn in the bioreactor; it can also be used in culturing experiments that do not require the bioreactor. The BSTC comprises four incubation/refrigeration chambers individually set at 4 to 50 degreesC (near-freezing to above body temperature). Each chamber holds three rugged tissue chamber modules (12 total), clear Teflon bags holding 30 ml of growth media, all positioned by a metal frame. Every 7 to 21 days (depending on growth rates), an astronaut uses a shrouded syringe and the bags' needleless injection ports to transfer a few cells to a fresh media bag, and to introduce a fixative so that the cells may be studied after flight. The design also lets the crew sample the media to measure glucose, gas, and pH levels, and to inspect cells with a microscope. The controller is monitored by the flight crew through a 23-cm (9-inch) color computer display on the face of the BSTC. This view shows the BTSC with the front panel open. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators.
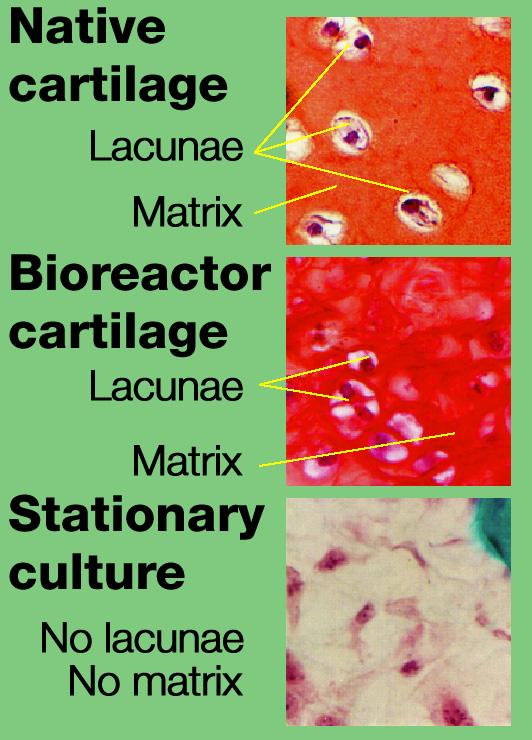
Dr. Lisa E. Freed of the Massachusetts Institute of Technology and her colleagues have reported that initially disc-like specimens tend to become spherical in space, demonstrating that tissues can grow and differentiate into distinct structures in microgravity. The Mir Increment 3 (Sept. 16, 1996 - Jan. 22, 1997) samples were smaller, more spherical, and mechanically weaker than Earth-grown control samples. These results demonstrate the feasibility of microgravity tissue engineering and may have implications for long human space voyages and for treating musculoskeletal disorders on earth. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators.

Leland W. K. Chung (left), Director, Molecular Urology Therapeutics Program at the Winship Cancer Institute at Emory University, is principal investigator for the NASA bioreactor demonstration system (BDS-05). With him is Dr. Jun Shu, an assistant professor of Orthopedics Surgery from Kuming Medical University China. The NASA Bioreactor provides a low turbulence culture environment which promotes the formation of large, three-dimensional cell clusters. Due to their high level of cellular organization and specialization, samples constructed in the bioreactor more closely resemble the original tumor or tissue found in the body. The Bioreactor is rotated to provide gentle mixing of fresh and spent nutrient without inducing shear forces that would damage the cells. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators. Credit: Emory University.
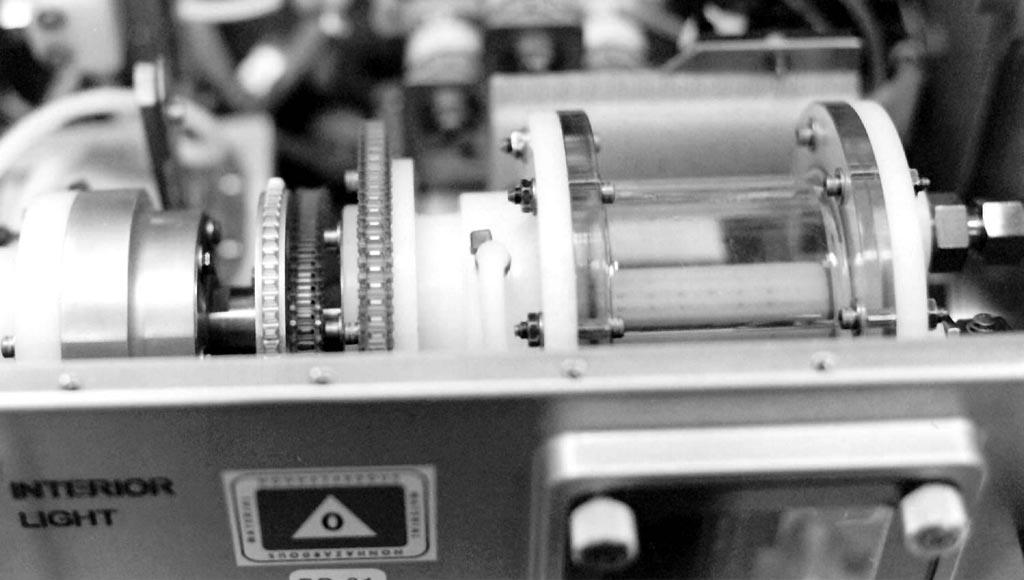
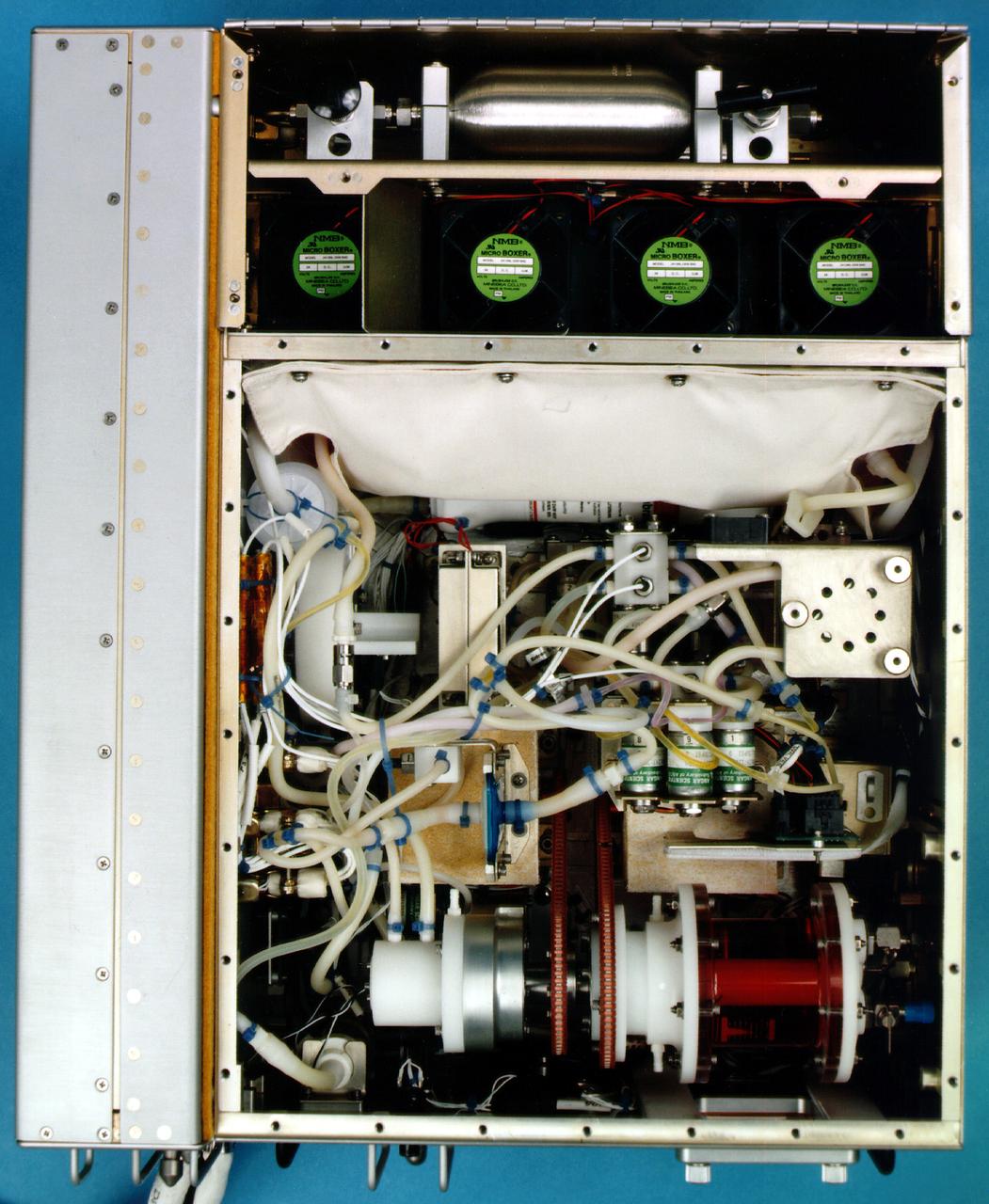
The bioreactor assembly within the flight unit.
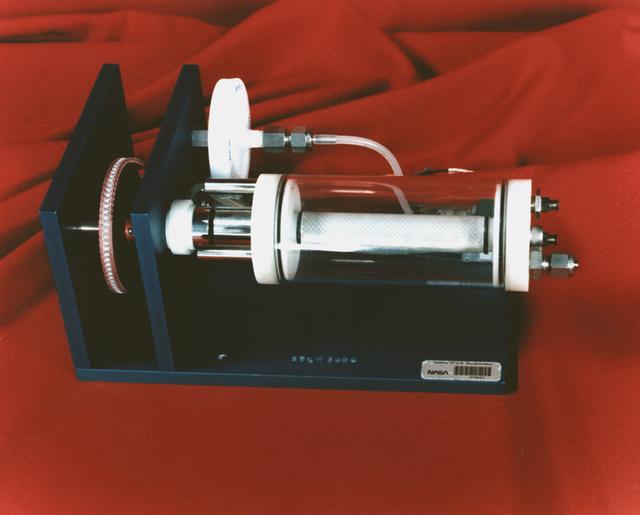
The NASA Bioreactor provides a low turbulence culture environment which promotes the formation of large, three-dimensional cell clusters. Due to their high level of cellular organization and specialization, samples constructed in the bioreactor more closely resemble the original tumor or tissue found in the body. NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues currently being cultured in rotating bioreactors by investigators
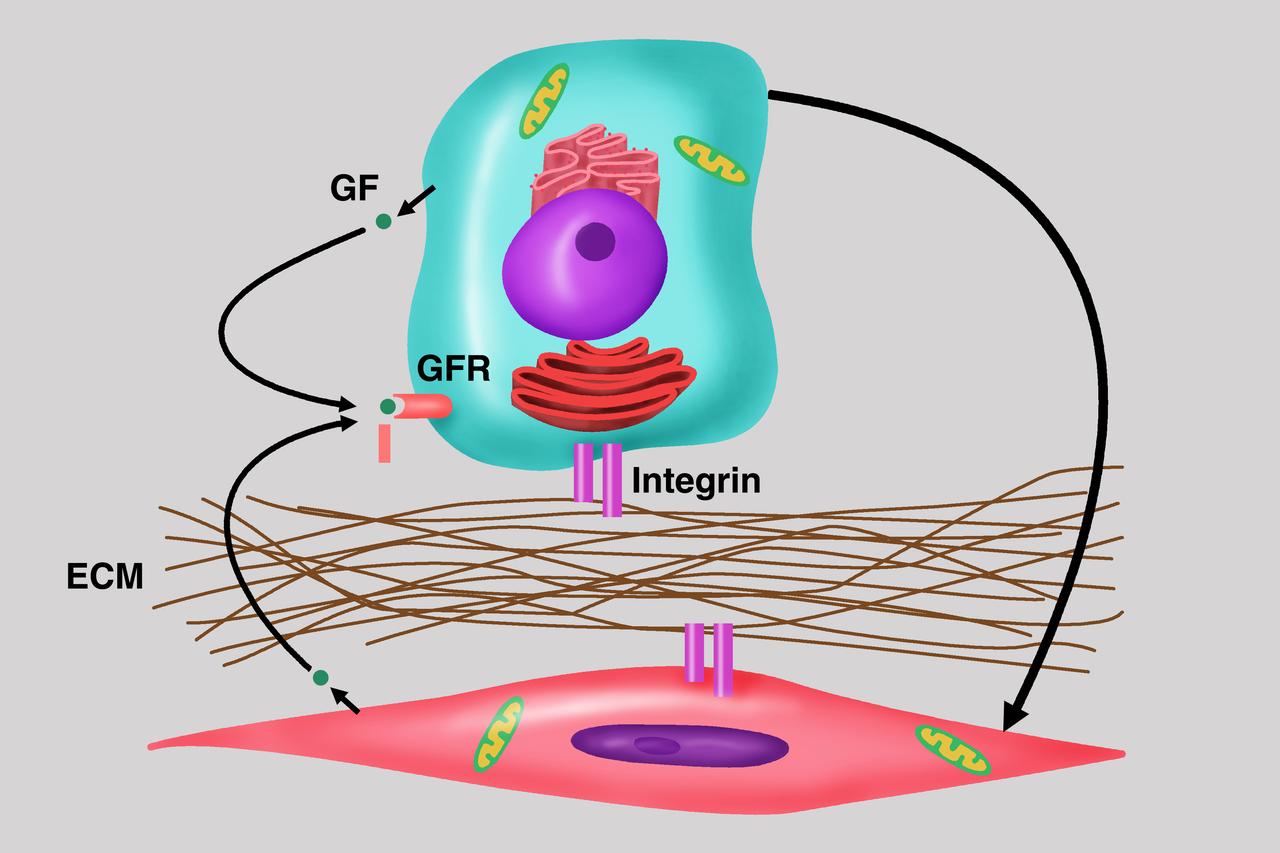
Diagram depicts the importance of cell-cell communication as central to the understanding of cancer growth and progression, the focus of the NASA bioreactor demonstration system (BDS-05) investigation. Microgravity studies will allow us to unravel the signaling and communication between these cells with the host and potential development of therapies for the treatment of cancer metastasis. The NASA Bioreactor provides a low turbulence culture environment which promotes the formation of large, three-dimensional cell clusters. Due to their high level of cellular organization and specialization, samples constructed in the bioreactor more closely resemble the original tumor or tissue found in the body. The Bioreactor is rotated to provide gentle mixing of fresh and spent nutrient without inducing shear forces that would damage the cells. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators. Credit: Emory University.

jsc2019e039818 (11/18/2018) --- Preflight imagery of six filled bioreactors from the BioRock project showing culture chambers containing growing microbes in media with basalt rocks. The purpose of the Biorock investigation is to examine the effects of altered gravity on the rock/microbe/liquid system as a whole. (Image Courtesy of: ESA)

jsc2019e039819 (7/26/2019) --- Photo documentation of Rosa Santomartino and Annemiek Waajen integrating a bioreactor for the BioRock experiment. The purpose of the Biorock investigation is to examine the effects of altered gravity on the rock/microbe/liquid system as a whole. (Image Courtesy of: ESA)
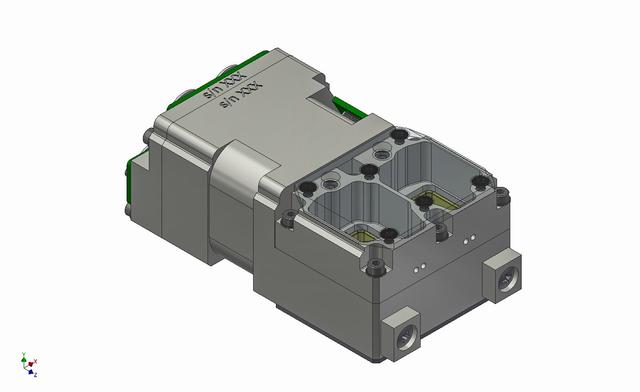
jsc2019e039822 (7/16/2019) --- A CAD image showing the structure of the bioreactor in the BioRock experiment.The two culture chambers are visible and the body of the unit, which contains media and fixative. (Image Courtesy of: ESA)
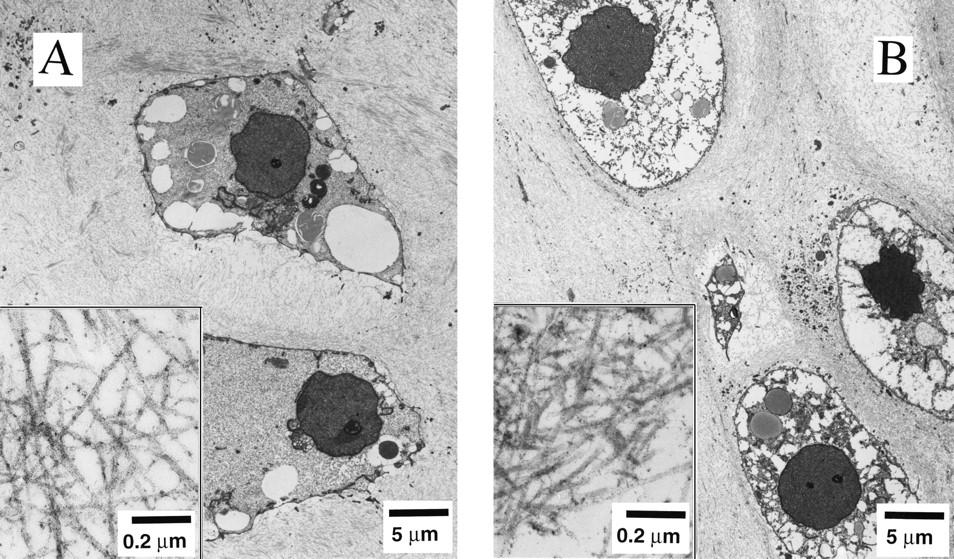
Dr. Lisa E. Freed of the Massachusetts Institute of Technology and her colleagues have reported that initially disc-like specimens tend to become spherical in space, demonstrating that tissues can grow and differentiate into distinct structures in microgravity. The Mir Increment 3 (Sept. 16, 1996 - Jan. 22, 1997) samples were smaller, more spherical, and mechanically weaker than Earth-grown control samples. These results demonstrate the feasibility of microgravity tissue engineering and may have implications for long human space voyages and for treating musculoskeletal disorders on earth. Final samples from Mir and Earth appeared histologically cartilaginous throughout their entire cross sections (5-8 mm thick), with the exception of fibrous outer capsules. Constructs grown on Earth (A) appeared to have a more organized extracellular matrix with more uniform collagen orientation as compared with constructs grown on Mir (B), but the average collagen fiber diameter was similar in the two groups (22 +- 2 nm) and comparable to that previously reported for developing articular cartilage. Randomly oriented collagen in Mir samples would be consistent with previous reports that microgravity disrupts fibrillogenesis. These are transmission electron micrographs of constructs from Mir (A) and Earth (B) groups at magnifications of x3,500 and x120,000 (Inset). The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Credit: Proceedings of the National Academy of Sciences.
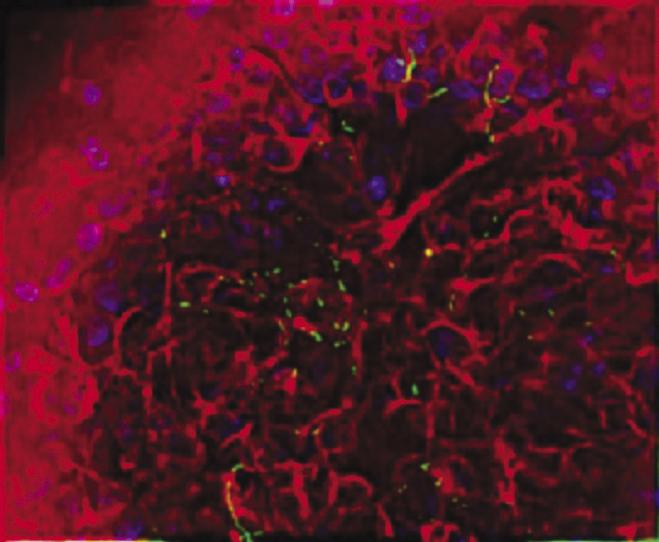
Salmonella typhimurium appears green in on human intestinal tissue (stained red) cultured in a NASA rotating wall bioreactor. Dr. Cheryl Nickerson of Tulane University is studying the effects of simulated low-g on a well-known pathogen, Salmonella typhimurium, a bacterium that causes two to four million cases of gastrointestinal illness in the United States each year. While most healthy people recover readily, S. typhimurium can kill people with weakened immune systems. Thus, a simple case of food poisoning could disrupt a space mission. Using the NASA rotating-wall bioreactor, Nickerson cultured S. typhimurium in modeled microgravity. Mice infected with the bacterium died an average of three days faster than the control mice, indicating that S. typhimurium's virulence was enhanced by the bioreactor. Earlier research showed that 3 percent of the genes were altered by exposure to the bioreactor. Nickerson's work earned her a 2001 Presidential Early Career Award for Scientists and Engineers.

ISS037-E-005692 (5 Oct. 2013) --- Russian cosmonaut Sergey Ryazanskiy, Expedition 37 flight engineer, prepares to manually mix samples in a Bioreactor for the CASKAD experiment in the Poisk Mini-Research Module 2 (MRM2) of the International Space Station.
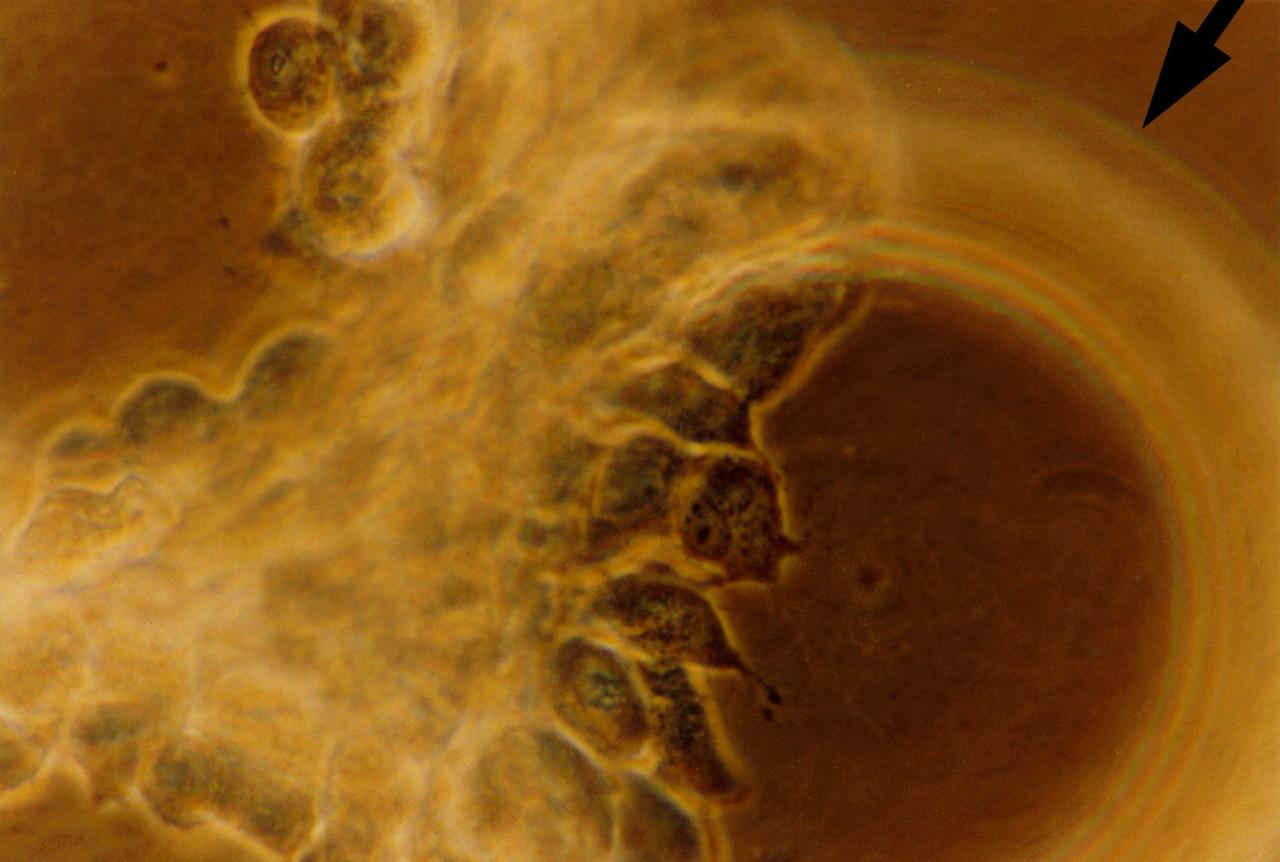
Human primary breast tumor cells after 49 days of growth in a NASA Bioreactor. Tumor cells aggregate on microcarrier beads (indicated by arrow). NASA's Marshall Space Flight Center (MSFC) is sponsoring research with Bioreactors, rotating wall vessels designed to grow tissue samples in space, to understand how breast cancer works. This ground-based work studies the growth and assembly of human mammary epithelial cell (HMEC) from breast cancer susceptible tissue. Radiation can make the cells cancerous, thus allowing better comparisons of healthy vs. tunorous tissue. Credit: Dr. Jearne Becker, University of South Florida

Dr. Cheryl Nickerson (right) of Tulane University is studying the effects of simulated low-g on a well-known pathogen, Salmonella typhimurium, a bacterium that causes two to four million cases of gastrointestinal illness in the United States each year. While most healthy people recover readily, S. typhimurium can kill people with weakened immune systems. Thus, a simple case of food poisoning could disrupt a space mission. Using the NASA rotating-wall bioreactor, Nickerson cultured S. typhimurium in modeled microgravity. Mice infected with the bacterium died an average of three days faster than the control mice, indicating that S. typhimurium's virulence was enhanced by the bioreactor. Earlier research showed that 3 percent of the genes were altered by exposure to the bioreactor. Nickerson's work earned her a 2001 Presidential Early Career Award for Scientists and Engineers.

Dr. Cheryl Nickerson of Tulane University is studying the effects of simulated low-g on a well-known pathogen, Salmonella typhimurium, a bacterium that causes two to four million cases of gastrointestinal illness in the United States each year. While most healthy people recover readily, S. typhimurium can kill people with weakened immune systems. Thus, a simple case of food poisoning could disrupt a space mission. Using the NASA rotating-wall bioreactor, Nickerson cultured S. typhimurium in modeled microgravity. Mice infected with the bacterium died an average of three days faster than the control mice, indicating that S. typhimurium's virulence was enhanced by the bioreactor. Earlier research showed that 3 percent of the genes were altered by exposure to the bioreactor. Nickerson's work earned her a 2001 Presidential Early Career Award for Scientists and Engineers.

Dr. Cheryl Nickerson of Tulane University is studying the effects of simulated low-g on a well-known pathogen, Salmonella typhimurium, a bacterium that causes two to four million cases of gastrointestinal illness in the United States each year. While most healthy people recover readily, S. typhimurium can kill people with weakened immune systems. Thus, a simple case of food poisoning could disrupt a space mission. Using the NASA rotating-wall bioreactor, Nickerson cultured S. typhimurium in modeled microgravity. Mice infected with the bacterium died an average of three days faster than the control mice, indicating that S. typhimurium's virulence was enhanced by the bioreactor. Earlier research showed that 3 percent of the genes were altered by exposure to the bioreactor. Nickerson's work earned her a 2001 Presidential Early Career Award for Scientists and Engineers.
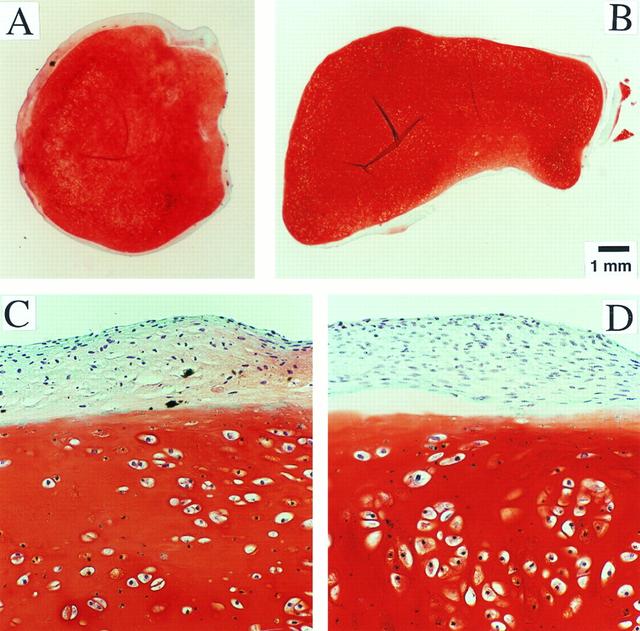
Dr. Lisa E. Freed of the Massachusetts Institute of Technology and her colleagues have reported that initially disc-like specimens of cartilage tend to become spherical in space, demonstrating that tissues can grow and differentiate into distinct structures in microgravity. The Mir Increment 3 (Sept. 16, 1996 - Jan. 22, 1997) samples were smaller, more spherical, and mechanically weaker than Earth-grown control samples. These results demonstrate the feasibility of microgravity tissue engineering and may have implications for long human space voyages and for treating musculoskeletal disorders on earth. Constructs grown on Mir (A) tended to become more spherical, whereas those grown on Earth (B) maintained their initial disc shape. These findings might be related to differences in cultivation conditions, i.e., videotapes showed that constructs floated freely in microgravity but settled and collided with the rotating vessel wall at 1g (Earth's gravity). In particular, on Mir the constructs were exposed to uniform shear and mass transfer at all surfaces such that the tissue grew equally in all directions, whereas on Earth the settling of discoid constructs tended to align their flat circular areas perpendicular to the direction of motion, increasing shear and mass transfer circumferentially such that the tissue grew preferentially in the radial direction. A and B are full cross sections of constructs from Mir and Earth groups shown at 10-power. C and D are representative areas at the construct surfaces enlarged to 200-power. They are stained red with safranin-O. NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). Photo credit: Proceedings of the National Academy of Sciences.
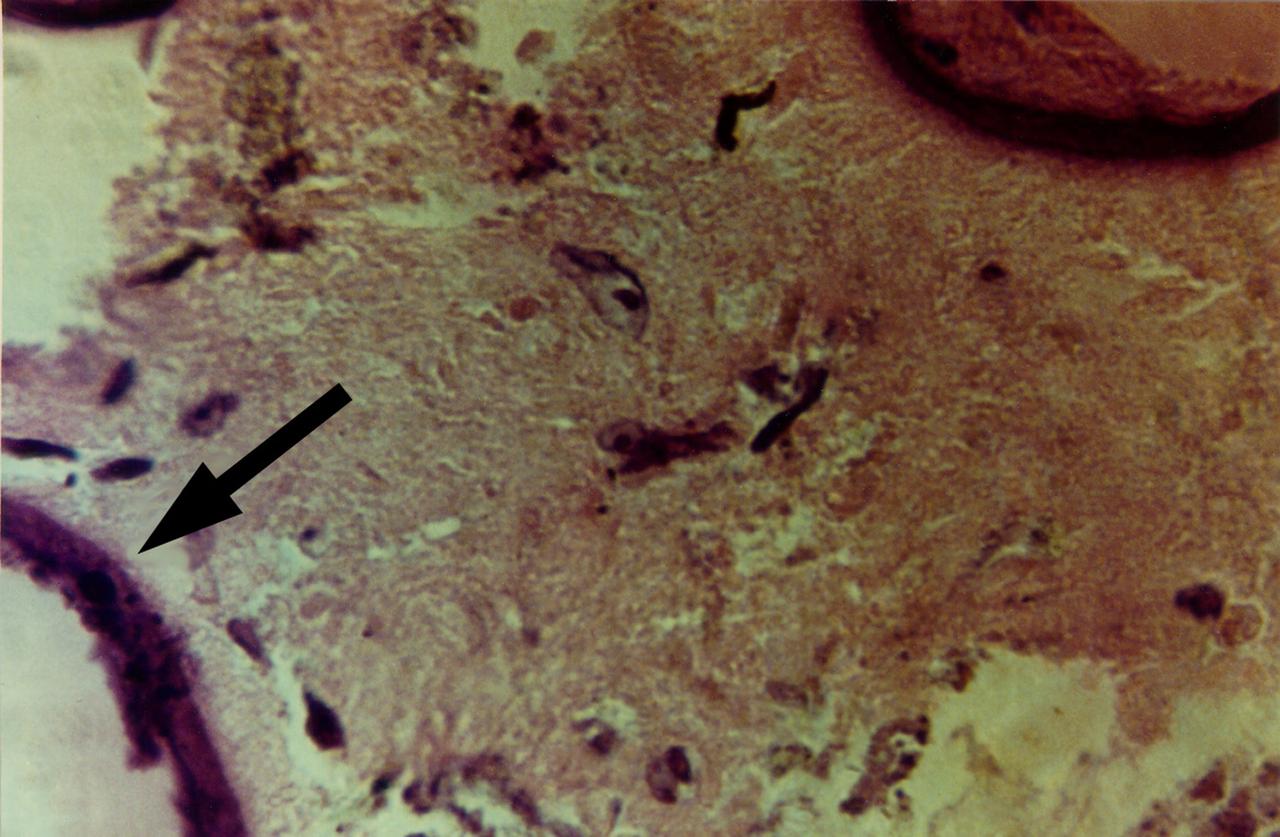
High magnification view of human primary breast tumor cells after 56 days of culture in a NASA Bioreactor. The arrow points to bead surface indicating breast cancer cells (as noted by the staining of tumor cell intermediate filaments). NASA's Marshall Space Flight Center (MSFC) is sponsoring research with Bioreactors, rotating wall vessels designed to grow tissue samples in space, to understand how breast cancer works. This ground-based work studies the growth and assembly of human mammary epithelial cell (HMEC) from breast cancer susceptible tissue. Radiation can make the cells cancerous, thus allowing better comparisons of healthy vs. tunorous tissue. Credit: Dr. Jearne Becker, University of South Florida
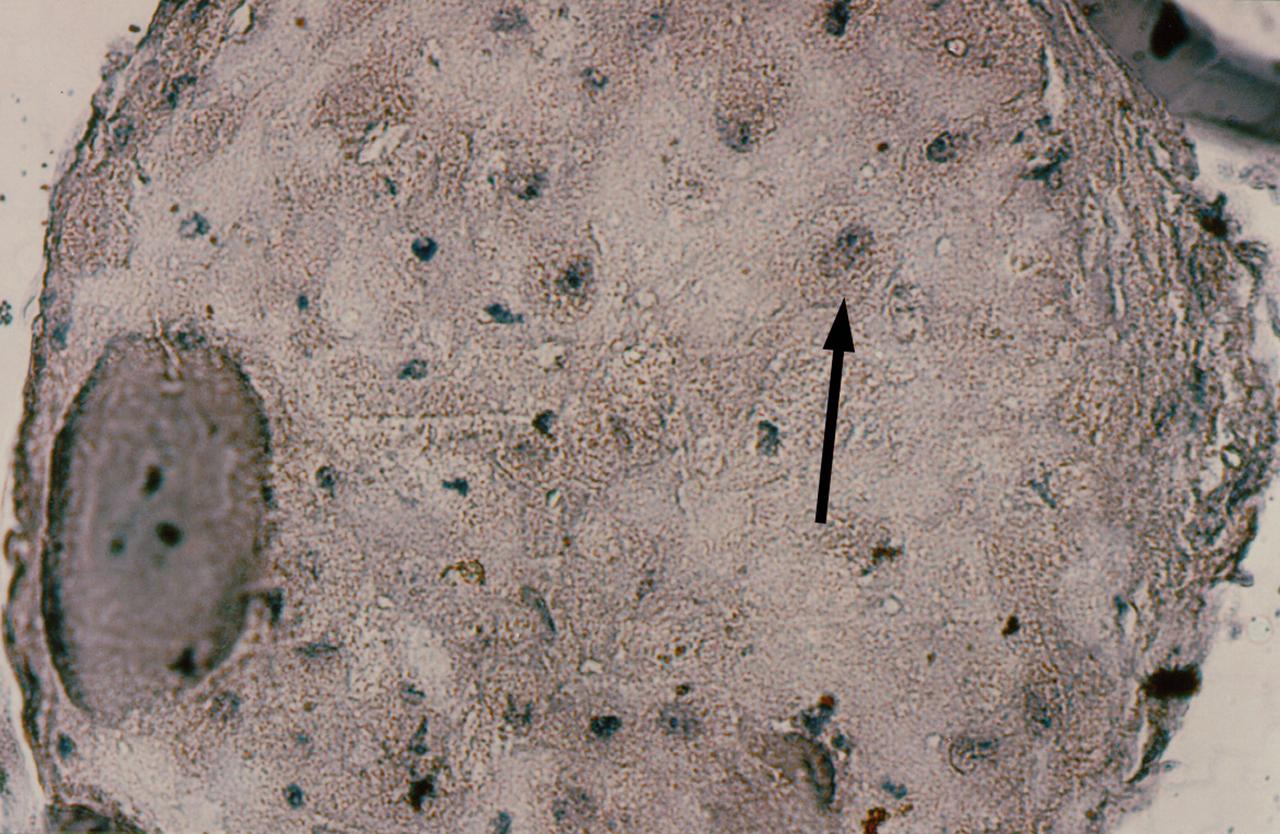
Human primary breast tumor cells after 56 days of culture in a NASA Bioreactor. A cross-section of a construct, grown from surgical specimens of brease cancer, stained for microscopic examination, reveals areas of tumor cells dispersed throughout the non-epithelial cell background. The arrow denotes the foci of breast cancer cells. NASA's Marshall Space Flight Center (MSFC) is sponsoring research with Bioreactors, rotating wall vessels designed to grow tissue samples in space, to understand how breast cancer works. This ground-based work studies the growth and assembly of human mammary epithelial cell (HMEC) from breast cancer susceptible tissue. Radiation can make the cells cancerous, thus allowing better comparisons of healthy vs. tunorous tissue. Credit: Dr. Jearne Becker, University of South Florida
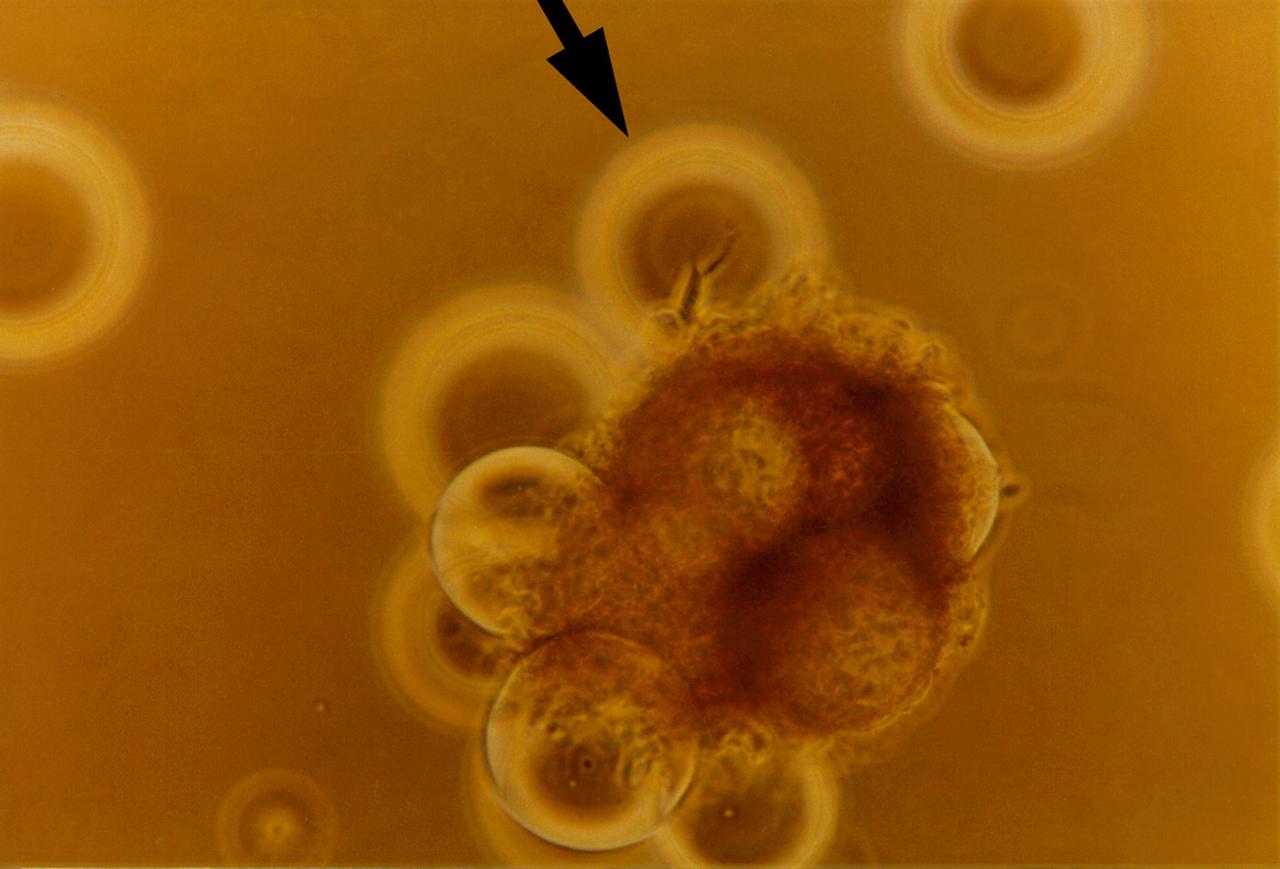
High magnification of view of tumor cells aggregate on microcarrier beads, illustrting breast cells with intercellular boundaires on bead surface and aggregates of cells achieving 3-deminstional growth outward from bead after 56 days of culture in a NASA Bioreactor. NASA's Marshall Space Flight Center (MSFC) is sponsoring research with Bioreactors, rotating wall vessels designed to grow tissue samples in space, to understand how breast cancer works. This ground-based work studies the growth and assembly of human mammary epithelial cell (HMEC) from breast cancer susceptible tissue. Radiation can make the cells cancerous, thus allowing better comparisons of healthy vs. tunorous tissue. Credit: Dr. Jearne Becker, University of South Florida.

jsc2019e039823 (7/19/2019) --- A CAD image showing the structure of the bioreactor in the BioRock experiment within its experimental container. The two culture chambers are visible along with the body of the unit, which contains media and fixative. (Image Courtesy of: ESA)

Dr. Harry Mahtani analyzes the gas content of nutrient media from Bioreactor used in research on human breast cancer. NASA's Marshall Space Flight Center (MSFC) is sponsoring research with Bioreactors, rotating wall vessels designed to grow tissue samples in space, to understand how breast cancer works. This ground-based work studies the growth and assembly of human mammary epithelial cells (HMEC) from breast cancer susceptible tissue. Radiation can make the cells cancerous, thus allowing better comparisons of healthy vs. tunourous tissues.

Dr. Robert Richmond extracts breast cell tissue from one of two liquid nitrogen dewars. NASA's Marshall Space Flight Center (MSFC) is sponsoring research with Bioreactors, rotating wall vessels designed to grow tissue samples in space, to understand how breast cancer works. This ground-based work studies the growth and assembly of human mammary epithelial cells (HMEC) from breast cancer susceptible tissue. Radiation can make the cells cancerous, thus allowing better comparisons of healthy vs. tunourous tissues.

S91-40049 (27 June 1991) --- JSC technician Tacey Prewitt checks the progress on a bioreactor experiment in JSC's Life Sciences Laboratory Bldg 37 biotechnology laboratory. Similar hardware is scheduled for testing aboard Atlantis, Orbiter Vehicle (OV) 104, during STS-44. Detailed Supplementary Objective (DSO) 316 Bioreactor/Flow and Particle Trajectory in Microgravity will checkout the rotating wall vessel hardware and hopefully will confirm researchers' theories and calculations about how flow fields work in space. Plastic beads of various sizes rather than cell cultures are being flown in the vessel for the STS-44 test.

Engineering mockup shows the general arrangement of the plarned Biotechnology Facility inside an EXPRESS rack aboard the International Space Station. This layout includes a gas supply module (bottom left), control computer and laptop interface (bottom right), two rotating wall vessels (top right), and support systems.

Robyn Gatens, left, deputy director, ISS Division and system capability leader for Environmental Control and Life Support Systems (ECLSS) at NASA Headquarters in Washington, tours laboratories in the Space Station Processing Facility at the agency's Kennedy Space Center in Florida, on June 13, 2018. To her right is Molly Anderson, deputy ECLSS capability lead at Johnson Space Center in Houston. They are viewing plant growth chambers and seeing firsthand some of the capabilities in the center's Exploration Research and Technology Programs.

Robyn Gatens, left, deputy director, ISS Division and system capability leader for Environmental Control and Life Support Systems (ECLSS) at NASA Headquarters in Washington, tours laboratories in the Space Station Processing Facility at the agency's Kennedy Space Center in Florida, on June 13, 2018. Standing behind her is Ralph Fritsche, long-duration food production project manager at Kennedy. Gatens is viewing plant growth chambers and seeing firsthand some of the capabilities in the center's Exploration Research and Technology Programs.

Inside a laboratory in the Neil Armstrong Operations and Checkout Building at NASA's Kennedy Space Center in Florida, Dr. Luke Roberson, right, principal investigator for research and development in Swamp Works, explains the algae bio reactor to Robyn Gatens, center, deputy director, ISS Division and system capability leader for Environmental Control and Life Support Systems (ECLSS) at NASA Headquarters in Washington, on June 13, 2018. At far left is Molly Anderson, deputy ECLSS capability lead at Johnson Space Center in Houston. They are seeing firsthand some of the capabilities in the center's Exploration Research and Technology Programs.
STS-131 payload; Ames Space Bio-Sciences Lab - Animal Enclosure Module (AEM) Space Tissue Loss Experiment Bioreactor - Cell Bioreactor, Stem Cell & Immune Experiement
The NASA Bioreactor provides a low turbulence culture environment which promotes the formation of large, three-dimensional cell clusters. Due to their high level of cellular organization and specialization, samples constructed in the bioreactor more closely resemble the original tumor or tissue found in the body. NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues currently being cultured in rotating bioreactors by investigators.
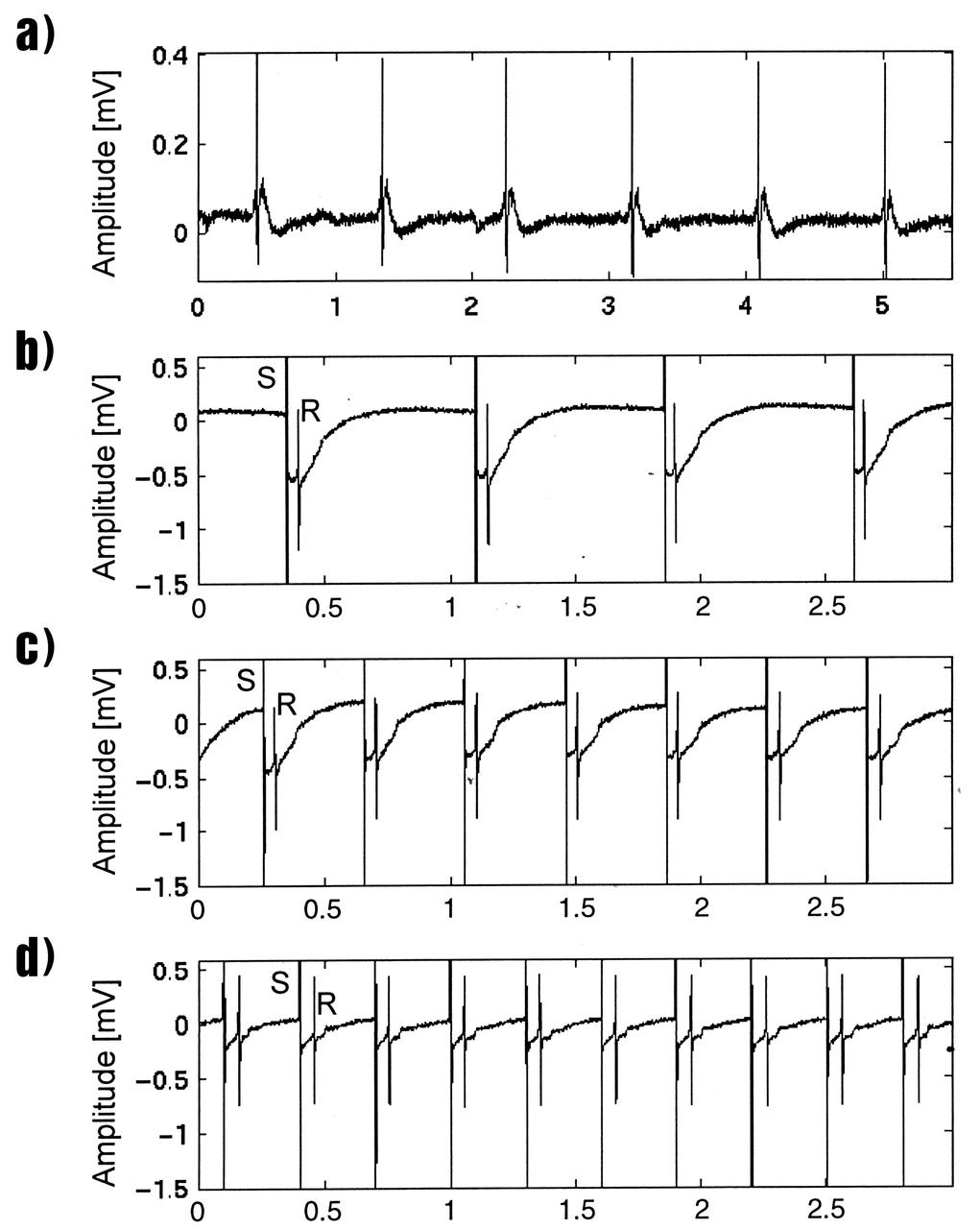
Lisa Freed and Gordana Vunjak-Novakovic, both of the Massachusetts Institute of Technology (MIT), have taken the first steps toward engineering heart muscle tissue that could one day be used to patch damaged human hearts. Cells isolated from very young animals are attached to a three-dimensional polymer scaffold, then placed in a NASA bioreactor. The cells do not divide, but after about a week start to cornect to form a functional piece of tissue. Functionally connected heart cells that are capable of transmitting electrical signals are the goal for Freed and Vunjak-Novakovic. Electrophysiological recordings of engineered tissue show spontaneous contractions at a rate of 70 beats per minute (a), and paced contractions at rates of 80, 150, and 200 beats per minute respectively (b, c, and d). The NASA Bioreactor provides a low turbulence culture environment which promotes the formation of large, three-dimensional cell clusters. The Bioreactor is rotated to provide gentle mixing of fresh and spent nutrient without inducing shear forces that would damage the cells. Due to their high level of cellular organization and specialization, samples constructed in the bioreactor more closely resemble the original tumor or tissue found in the body. NASA-sponsored bioreactor research has been instrumental in helping scientists to better understand normal and cancerous tissue development. In cooperation with the medical community, the bioreactor design is being used to prepare better models of human colon, prostate, breast and ovarian tumors. Cartilage, bone marrow, heart muscle, skeletal muscle, pancreatic islet cells, liver and kidney are just a few of the normal tissues being cultured in rotating bioreactors by investigators. The work is sponsored by NASA's Office of Biological and Physical Research. The bioreactor is managed by the Biotechnology Cell Science Program at NASA's Johnson Space Center (JSC). Credit: NASA and MIT.
jsc2019e039820 (12/10/2018) --- Preflight imagery of the two culture chambers of a single experimental unit in the BioRock experiment. The purpose of the Biorock investigation is to examine the effects of altered gravity on the rock/microbe/liquid system as a whole. (Image Courtesy of: ESA)
STS-131 payload; Ames Space Bio-Sciences Lab, Dr Eduardo Almeida P. I.; Cell Bioreactors with media syringes attached

STS-131 payload; Ames Space Bio-Sciences Lab, Dr Eduardo Almeida P. I.; complete cell bioreactor prior to connecting to rail

STS-131 payload; Ames Space Bio-Sciences Lab, Dr Eduardo Almeida P. I.; hollow fibers used in Cell Bioreactors

Time-lapse exposure depicts Bioreactor rotation. NASA's Marshall Space Flight Center (MSFC) is sponsoring research with Bioreactors, rotating wall vessels designed to grow tissue samples in space, to understand how breast cancer works. This ground-based work studies the growth and assembly of human mammary epithelial cells (HMEC) from breast cancer susceptible tissue. Radiation can make the cells cancerous, thus allowing better comparisons of healthy vs. tunourous tissues.
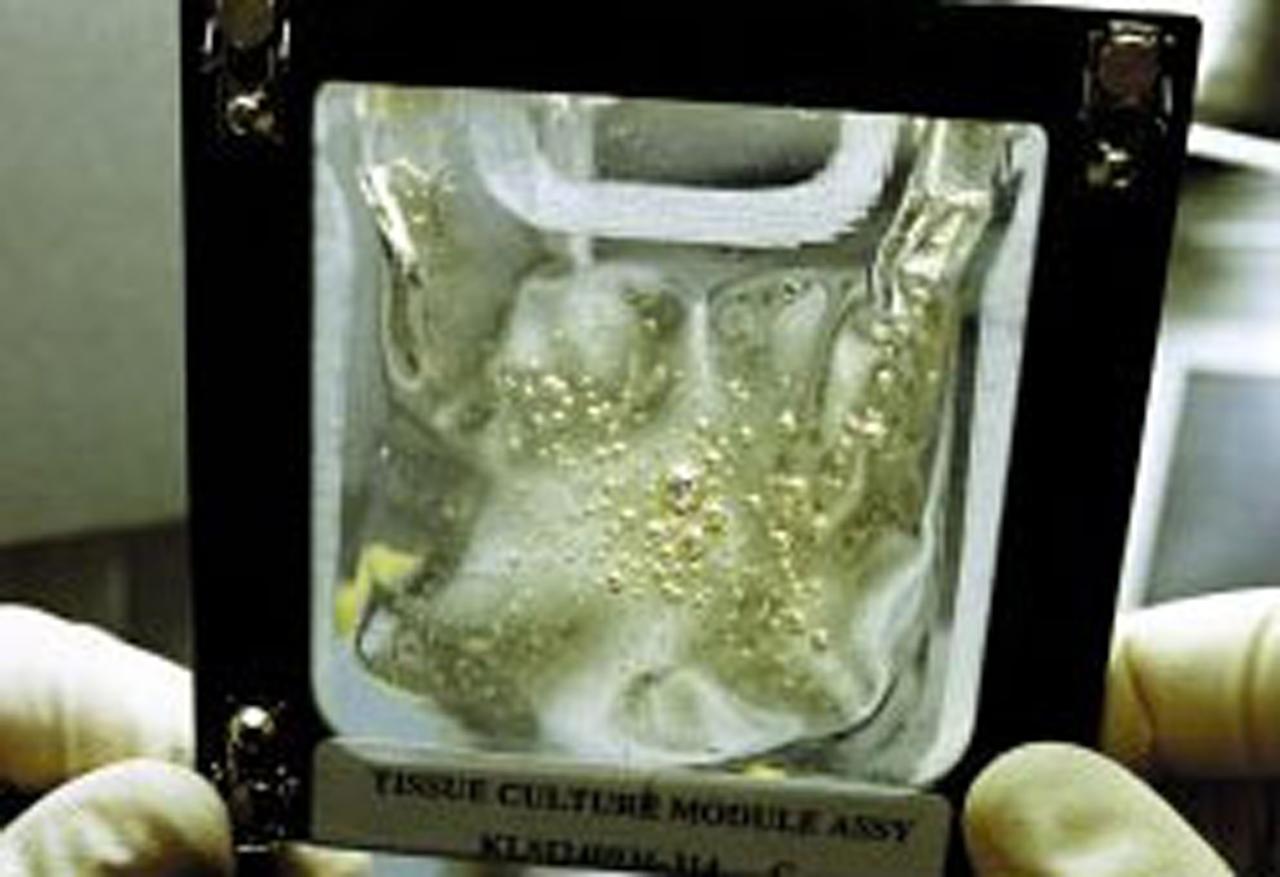
During the STS-90 shuttle flight in April 1998, cultured renal cortical cells revealed new information about genes. Timothy Hammond, an investigator in NASA's microgravity biotechnology program was interested in culturing kidney tissue to study the expression of proteins useful in the treatment of kidney diseases. Protein expression is linked to the level of differentiation of the kidney cells, and Hammond had difficulty maintaining differentiated cells in vitro. Intrigued by the improvement in cell differentiation that he observed in rat renal cells cultured in NASA's rotating wall vessel (a bioreactor that simulates some aspects of microgravity) and during an experiment performed on the Russian Space Station Mir, Hammond decided to sleuth out which genes were responsible for controlling differentiation of kidney cells. To do this, he compared the gene activity of human renal cells in a variety of gravitational environments, including the microgravity of the space shuttle and the high-gravity environment of a centrifuge. Hammond found that 1,632 genes out of 10,000 analyzed changed their activity level in microgravity, more than in any of the other environments. These results have important implications for kidney research as well as for understanding the basic mechanism for controlling cell differentiation.
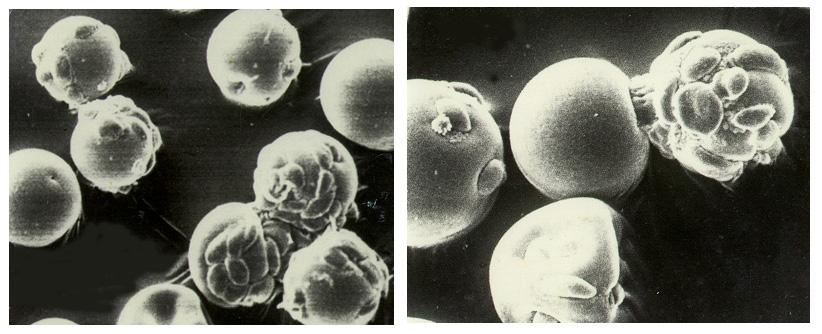
Biomedical research offers hope for a variety of medical problems, from diabetes to the replacement of damaged bone and tissues. Bioreactors, which are used to grow cells and tissue cultures, play a major role in such research and production efforts. Anchorage dependent cells on STS-95 will be grown on beads similar to these cells produced during previous investigations. Recombinant proteins may offer the possibility of reducing or eliminating transplant rejections. Research by Synthecon, Inc. using the BioDyn Bioreactor will focus on the preliminary process for growing a proprietary recombinant protein that can decrease rejection of transplanted tissue. The cells producing this protein are anchorage dependent, meaning that they must attach to something to grow. These cells will be cultured in the bioreactor in a medium containing polymer microbeads. Synthecon hopes that the data from this mission will lead to the development of a commercial protein that will aid in prevention of transplant rejection.
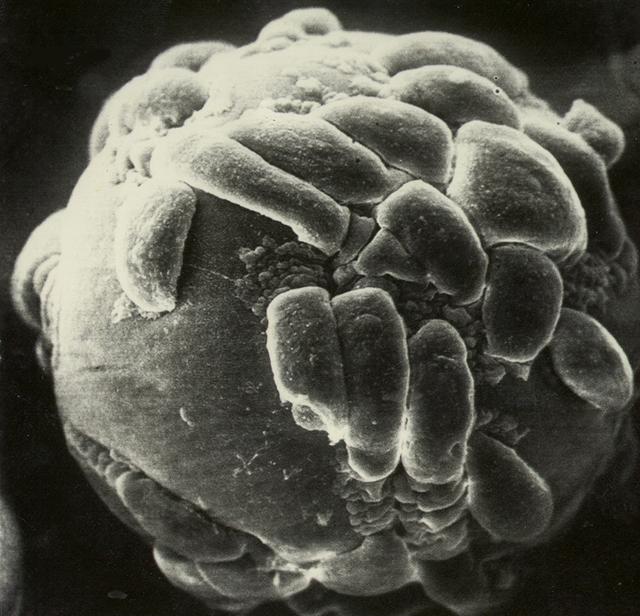
Biomedical research offers hope for a variety of medical problems, from diabetes to the replacement of damaged bone and tissues. Bioreactors, which are used to grow cells and tissue cultures, play a major role in such research and production efforts. Anchorage dependent cells on STS-95 will be grown on beads, similar to these cells produced during previous investigations. Recombinant proteins may offer the possibility of reducing or eliminating transplant rejections. Research by Synthecon, Inc. using the BioDyn Bioreactor will focus on the preliminary process for growing a proprietary recombinant protein that can decrease rejection of transplanted tissue. The cells producing this protein are anchorage dependent, meaning that they must attach to something to grow. These cells will be cultured in the bioreactor in a medium containing polymer microbeads. Synthecon hopes that the data from this mission will lead to the development of a commercial protein that will aid in prevention of transplant rejection.

jsc2021e031154 (7/22/2021) --- A preflight photo of the BioCell bioreactor for housing engineered muscle. Developed in collaboration with BioServe Space Technologies. Part of Cardinal Muscle investigation. Photo courtesy of the Palo Alto Veterans Institute for Research.

STS085-336-018 (7 - 19 August 1997) --- Astronaut Robert L. Curbeam, Jr., mission specialist, with a Bioreactor Demonstration System (BDS-03) specimen on the mid-deck of the Space Shuttle Discovery.

STS-131 payload; Ames Space Bio-Sciences Lab, Dr Eduardo Almeida P. I.; Scientists prepare cell bioreactors within glove box at Kennedy Space Center (KSC)

S85-E-5011 (9 August 1997) --- Astronaut Robert L. Curbeam, Jr., mission specialist, works with the Bioreactor Demonstration System (BDS) on the Space Shuttle Discovery's mid-deck.
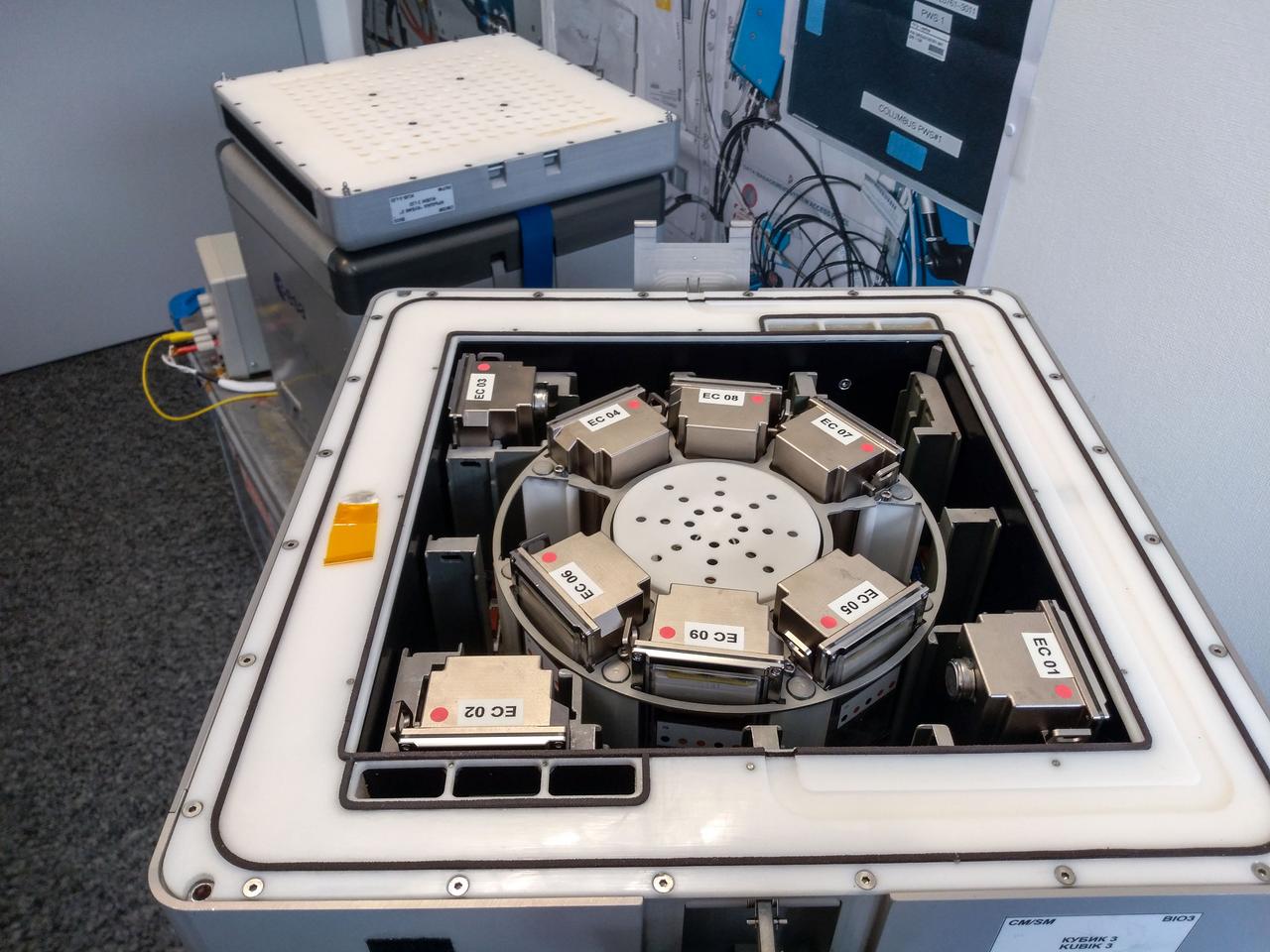
jsc2019e039824 (4/3/2019) --- Preflight imagery showing nine BioRock experimental containers each containing a bioreactor in the KUBIK centrifuge. The KUBIK will be used to simulate Martian and terrestrial gravity. The purpose of the Biorock investigation is to examine the effects of altered gravity on the rock/microbe/liquid system as a whole. (Image Courtesy of: ESA)

iss067e191207 (7/22/2022) --- A view of the of a Plate Habitat (PHAB) at -20°C after insertion into the SABL incubator aboard the International Space Station (ISS). The goal of the Protein Manufacturing project is to demonstrate the use of a novel bioreactor technology for growing high-protein food on the International Space Station (ISS).
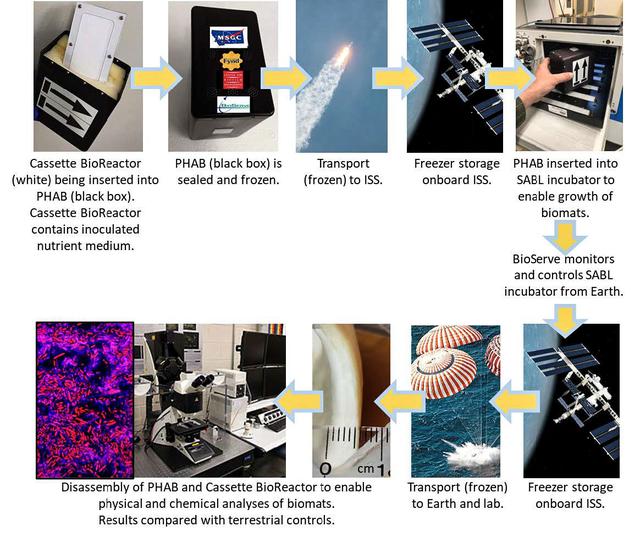
jsc2022e031226 (4/26/2022) --- A mission overview of the Protein Manufacturing investigation shows hardware, operations, and scientific details. The Protein Manufacturing project demonstrates and tests the operation of a novel bioreactor technology to support robust fungal growth for the production of high-protein food in a low-Earth orbit, space environment. Image courtesy of BioServe.

iss067e191182 (7/22/2022) --- A view of the of a Plate Habitat (PHAB) at -20°C prior to insertion into the SABL incubator aboard the International Space Station (ISS). The goal of the Protein Manufacturing project is to demonstrate the use of a novel bioreactor technology for growing high-protein food on the International Space Station (ISS).

NASA's Marshall Space Flight Center (MSFC) is sponsoring research with Bioreactors, rotating wall vessels designed to grow tissue samples in space, to understand how breast cancer works. This ground-based work studies the growth and assembly of human mammary epithelial cells (HMEC) from breast cancer susceptible tissue. Radiation can make the cells cancerous, thus allowing better comparisons of healthy vs. tunourous tissues. Here, two High-Aspect Ratio Vessels turn at about 12 rmp to keep breast tissue constructs suspended inside the culture media. Syringes allow scientists to pull for analysis during growth sequences. The tube in the center is a water bubbler that dehumidifies the air to prevent evaporation of the media and thus the appearance of destructive bubbles in the bioreactor.

Dr. Timothy G. Hammond of the Department of Internal Medicine, Nephrology Section, Tulane University Medical Center, New Orleans, LA, is one of NASA's principal investigators conducting research with the NASA Bioreactor project directed by Johrnson Space Center. Hammond's investigations include Production of 1-25- diOH D3 by Renal Epithelial Cells in Simulated Microgravity Culture and Differentiation of Cultured Normal Human Renal Epithelial Cells in Microgravity. Photo credit: Tulane University.

STS059-35-023 (9-20 April 1994) --- Astronaut Kevin P. Chilton, pilot, works with an advanced cell bioreactor, which incorporated the first ever videomicroscope, Space Tissue Loss (STL-B), on the Space Shuttle Endeavour's middeck. This experiment studied cell growth during the STS-59 mission. Chilton was joined in space by five other NASA astronauts for a week and a half of support to the Space Radar Laboratory (SRL-1) mission and other tasks.

iss067e191193_alt (7/22/2022) --- NASA astronaut Jessica Watkins holding a Plate Habitat (PHAB) at -20°C prior to insertion into the Space Automated Bioproduct Laboratory (SABL) incubator aboard the International Space Station (ISS). The goal of the Protein Manufacturing project is to demonstrate the use of a novel bioreactor technology for growing high-protein food on the International Space Station (ISS).
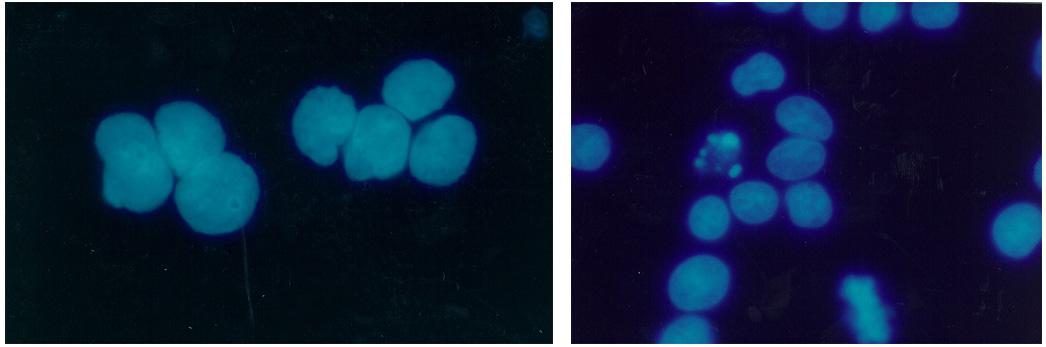
Biomedical research offers hope for a variety of medical problems, from diabetes to the replacement of damaged bone and tissues. Bioreactors, which are used to grow cells and tissue cultures, play a major role in such research and production efforts. The objective of the research was to define a way to differentiate between effects due to microgravity and those due to possible stress from non-optimal spaceflight conditions.
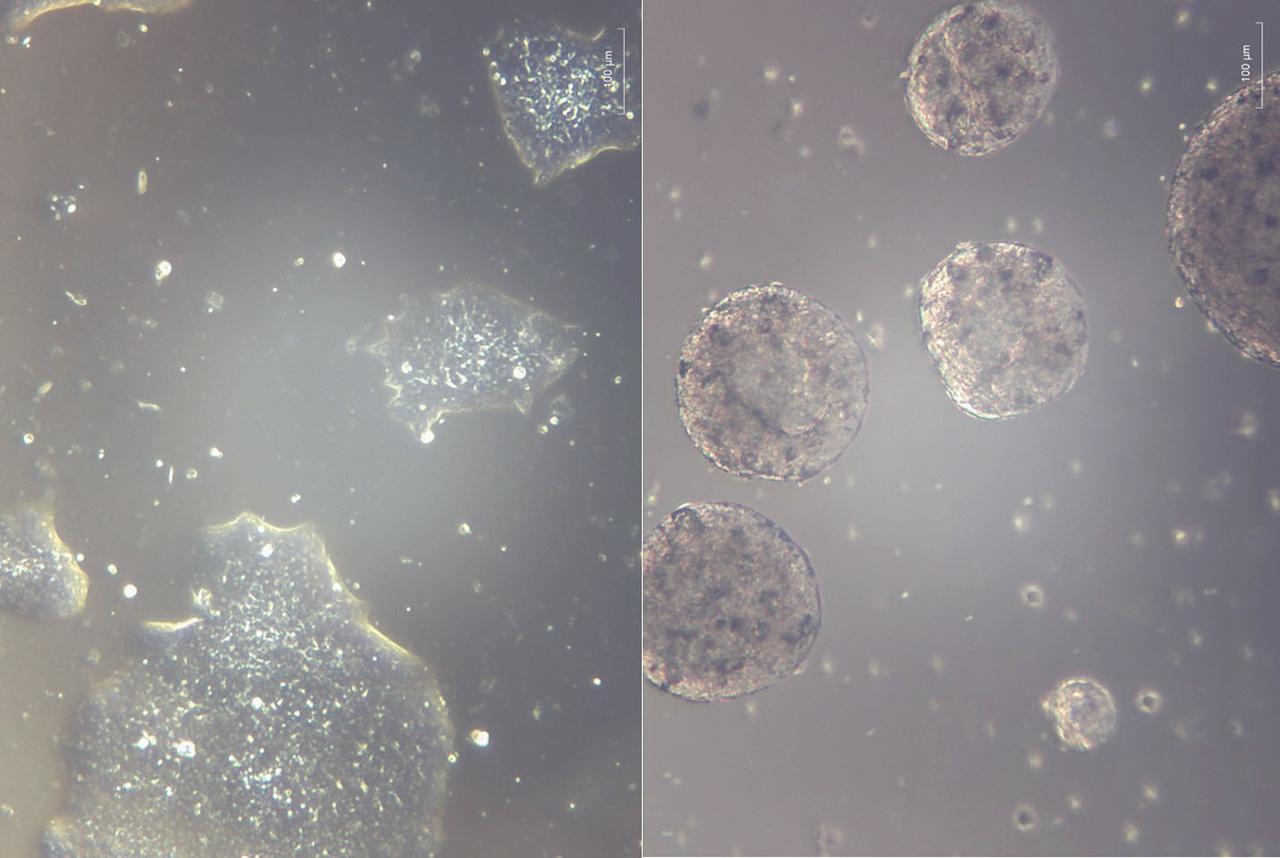
jsc2025e007240 (2/18/2025) ---Induced pluripotent stem cells (iPSCs) grown attached as a mat in 2D on Earth as the current state-of-the-art (left) vs. iPSC experimentally grown as spheres in suspension in 3D bioreactors on Earth in simulated microgravity conditions (right). In-Space Production of Induced Pluripotent Stem Cells for Clinical Application (StemCellEX-IP1) investigation aims to use microgravity to expand and mass-produce high quality human iPSCs. Image courtesy of BioServe.

jsc2022e031234 (4/26/2022) --- A preflight view of Bioreactors enclosed in experiment containers and interfaced with a system simulating on ground Kubik incubator located on board the International Space Station. The PROtection MEdiated by antioxidant nanoTEchnOlogy against neuronal damage in space (PROMETEO) (Antioxidant Protection) investigation proposes the use of biocompatible and biodegradable polydopamine-based nanoparticles to provide antioxidant protection to neurons undergoing exposure to altered gravity and cosmic radiation. Image courtesy of Kayser Italia.
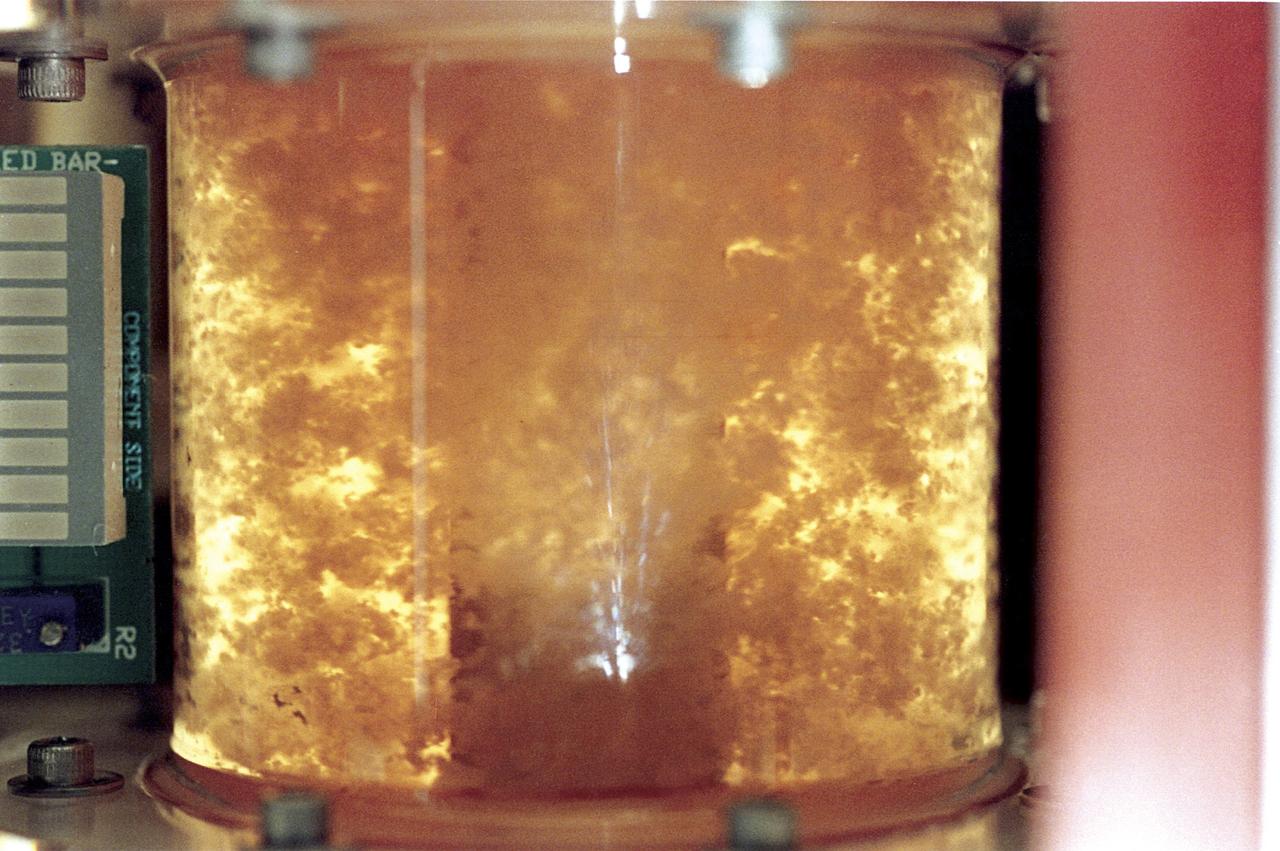
Within five days, bioreactor cultivated human colon cancer cells (shown) grown in Microgravity on the STS-70 mission in 1995, had grown 30 times the volume of the control specimens on Earth. The samples grown in space had a higher level of cellular organization and specialization. Because they more closely resemble tumors found in the body, microgravity grown cell cultures are ideal for research purposes.

jsc2021e031156 (7/22/2021) --- A photo of postdoctoral fellow, Bugra Ayan, PhD, exchanging the media of the engineered skeletal muscle bioreactor. Tissue Engineered Muscle in Microgravity as a Novel Platform to Study Sarcopenia (Cardinal Muscle) evaluates whether engineered human muscle cells cultured in microgravity are a valid model for studying muscle loss. Photo courtesy of the Palo Alto Veterans Institute for Research.

jsc2025e015699 (3/6/2025) --- The SOPHONSTER team with the bioreactor used to grow Chlorella sp. in ground studies. The SOPHie’s ON hamSTER (SOPHONSTER) investigation aims to cultivate microalgae (Chlorella sp.) in a space environment to study growth, protein content, and cell wall rigidity under microgravity conditions. Results could elucidate ways to improve plant-based, protein-rich alternatives to meat and dairy. Image courtesy of Sophie’s BioNutrients.

Breast tissue specimens in traditional sample dishes. NASA's Marshall Space Flight Center (MSFC) is sponsoring research with Bioreactors, rotating wall vessels designed to grow tissue samples in space, to understand how breast cancer works. This ground-based work studies the growth and assembly of human mammary epithelial cells (HMEC) from breast cancer susceptible tissue. Radiation can make the cells cancerous, thus allowing better comparisons of healthy vs. tunourous tissues.
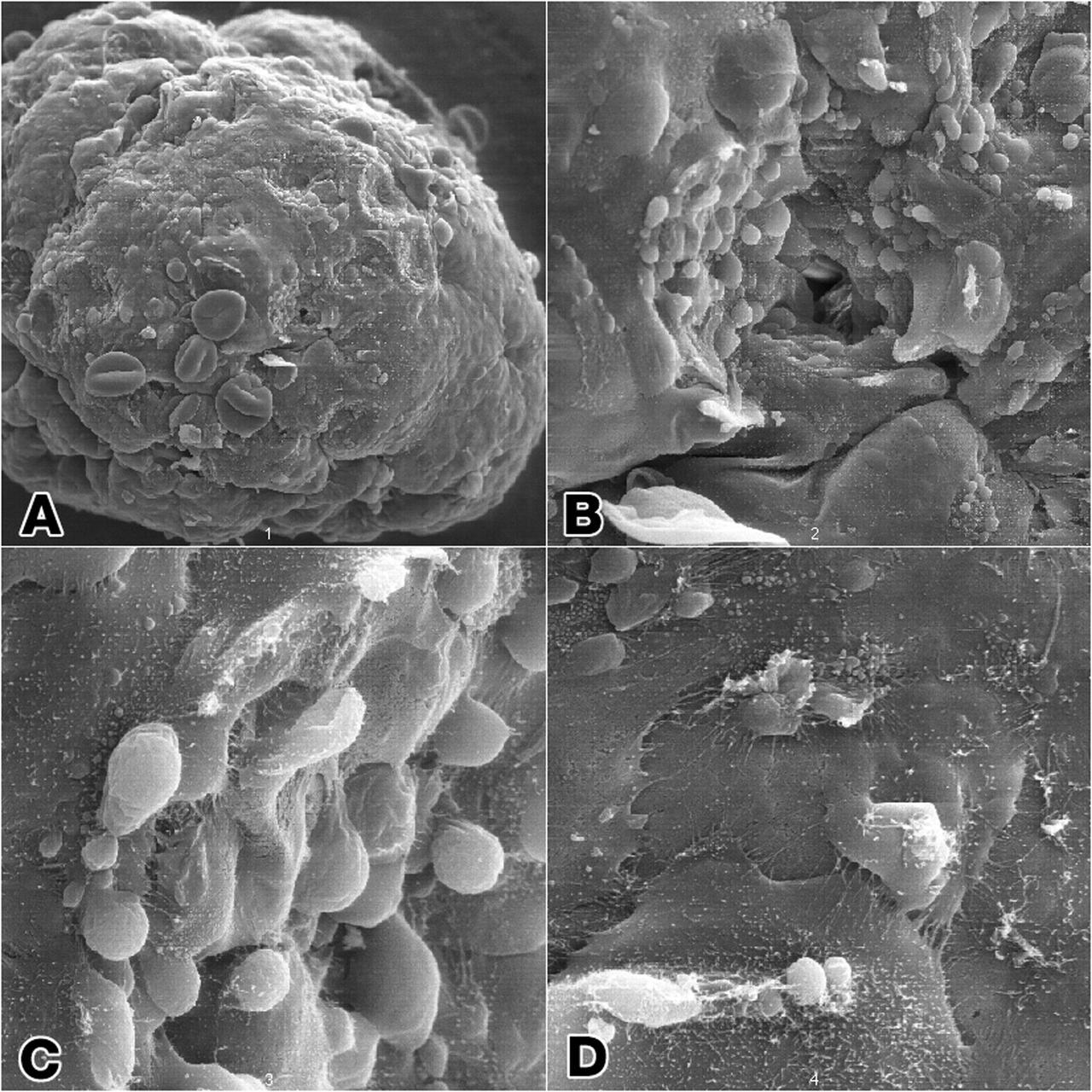
Epithelial cell monoculture: Long-term growth of human mammary epithelial cells (HMEC) grown in monoculture as 3-dimensional constructions in the presence of attachment beads in the NASA Bioreactor. A: A typical construct about 3.5 mm (less than 1/8th inch) in diameter with slightly dehydrted, crinkled beads contained on the surface as well as within the 3-dimensional structure. B: The center of these constructs is hollow. Crinkling of the beads causes a few to fall out, leaving crater-like impressiions in the construct. The central impression shows a small hole that accesses the hollow center of the construct. C: A closeup view of the cells and the hole the central impression. D: Closer views of cells in the construct showing sell-to-cell interactions. NASA's Marshall Space Flight Center (MSFC) is sponsoring research with Bioreactors, rotating wall vessels designed to grow tissue samples in space, to understand how breast cancer works. This ground-based work studies the growth and assembly of human mammary epithelial cell (HMEC) from breast cancer susceptible tissue. Radiation can make the cells cancerous, thus allowing better comparisons of healthy vs. tunorous tissue. Credit: Dr. Robert Richmond, NASA/Marshall Space Flight Center (MSFC).

STS070-301-025 (13-22 July 1995) --- Astronaut Mary Ellen Weber works with a syringe related to the Bioreactor Development System (BDS). The almost weightless state of space travel provides life science researchers with the opportunity to grow cells into three-dimensional tissue pieces that are not achievable using conventional tissue culture methods on Earth. At specified times during the STS-70 mission, crew members injected color producing substances to document fluid movement in the reactor, and various-sized beads to estimate the tissue size that could be supported in the Bioreactor. The photo was among NASA's first release of still photography from the STS-70 mission. The mission was launched from the Kennedy Space Center (KSC) on July 13, 1995, and ended when Discovery landed on Runway 33 there on July 22, 1995. The crew members were astronauts Terence T. (Tom) Henricks, commander; Kevin R. Kregel, pilot; and Donald A. Thomas, Nancy J. Currie and Weber, all mission specialists.
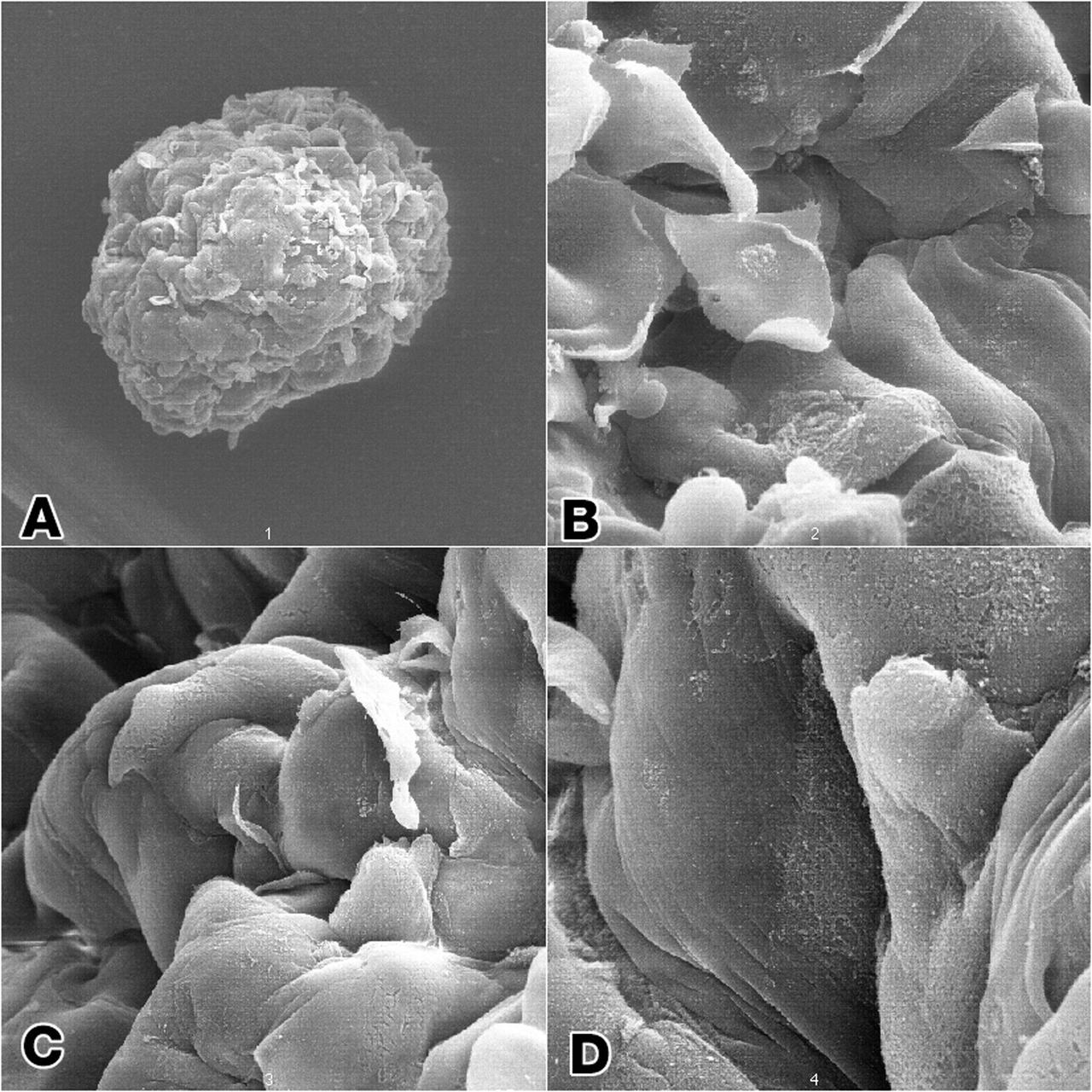
Epithelial and fibroblast cell coculture: Long-term growth human mammary epithelial cells (HMEC) admixed in coculture with fibroblast from the same initial breast tissue grown as 3-dimenstional constructions in the presence of attachment beads in the NASA Bioreactor. A: A typical constrct about 2.0 mm in diameter without beads on the surface. The center of these constrcts is hollow, and beads are organized about the irner surface. Although the coculture provides smaller constructs than the monoculture, the metabolic of the organized cells is about the same. B, C, D: Closer views of cells showing that the shape of cells and cell-to-cell interactions apprear different in the coculture than in the monoculture constructs. NASA's Marshall Space Flight Center (MSFC) is sponsoring research with Bioreactors, rotating wall vessels designed to grow tissue samples in space, to understand how breast cancer works. This ground-based work studies the growth and assembly of human mammary epithelial cell (HMEC) from breast cancer susceptible tissue. Radiation can make the cells cancerous, thus allowing better comparisons of healthy vs. tunorous tissue. Credit: Dr. Robert Richmond, NASA/Marshall Space Flight Center (MSFC).
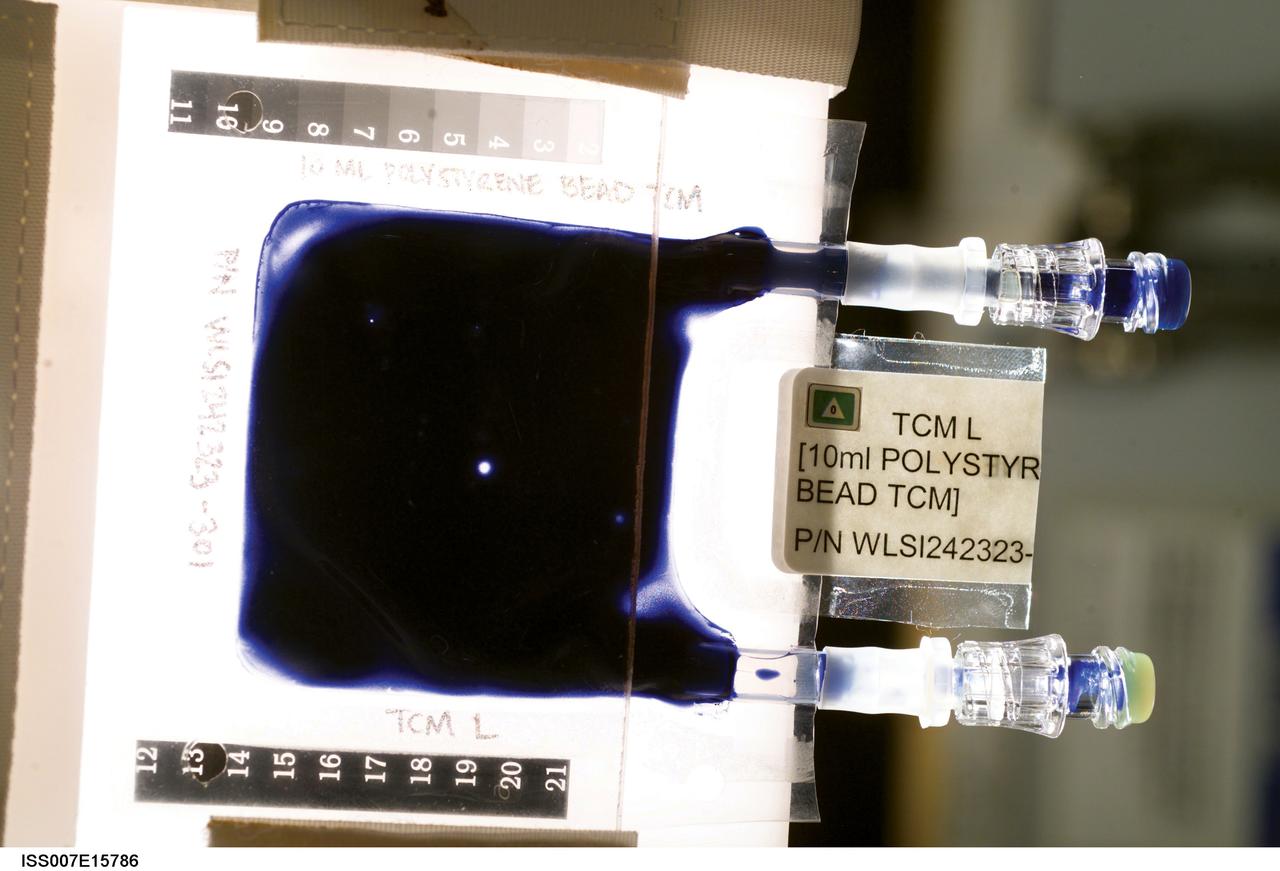
Aboard the International Space Station (ISS), the Tissue Culture Medium (TCM) is the bioreactor vessel in which cell cultures are grown. With its two syringe ports, it is much like a bag used to administer intravenous fluid, except it allows gas exchange needed for life. The TCM contains cell culture medium, and when frozen cells are flown to the ISS, they are thawed and introduced to the TCM through the syringe ports. In the Cellular Biotechnology Operations Support System-Fluid Dynamics Investigation (CBOSS-FDI) experiment, several mixing procedures are being assessed to determine which method achieves the most uniform mixing of growing cells and culture medium.

A prototype of Organic Processor Assembly (OPA) – technology capable of treating mixed organic wastes – arrives at the Neil Armstrong Operations and Checkout Building at NASA’s Kennedy Space Center in Florida on Aug. 19, 2020. At the heart of the OPA is an anaerobic membrane bioreactor – a hybrid technology that couples anaerobic digestion with membrane filtration. Developed through a collaboration between Kennedy’s Dr. Luke Roberson and the University of South Florida’s Dr. Daniel Yeh, the OPA was designed for an early planetary base scenario to help close the resource recovery loop, decreasing the agency’s dependence on resupply missions.

A prototype of Organic Processor Assembly (OPA) – technology capable of treating mixed organic wastes – arrives at the Neil Armstrong Operations and Checkout Building at NASA’s Kennedy Space Center in Florida on Aug. 19, 2020. Developed through a collaboration between Kennedy’s Dr. Luke Roberson and the University of South Florida’s Dr. Daniel Yeh, the OPA was designed for an early planetary base scenario to help close the resource recovery loop, decreasing the agency’s dependence on resupply missions. At the heart of the OPA is an anaerobic membrane bioreactor – a hybrid technology that couples anaerobic digestion with membrane filtration.

A prototype of Organic Processor Assembly (OPA) – technology capable of treating mixed organic wastes – arrives at the Neil Armstrong Operations and Checkout Building at NASA’s Kennedy Space Center in Florida on Aug. 19, 2020. At the heart of the OPA is an anaerobic membrane bioreactor – a hybrid technology that couples anaerobic digestion with membrane filtration. Developed through a collaboration between Kennedy’s Dr. Luke Roberson and the University of South Florida’s Dr. Daniel Yeh, the OPA was designed for an early planetary base scenario to help close the resource recovery loop, decreasing the agency’s dependence on resupply missions.

A prototype of Organic Processor Assembly (OPA) – technology capable of treating mixed organic wastes – arrives at the Neil Armstrong Operations and Checkout Building at NASA’s Kennedy Space Center in Florida on Aug. 19, 2020. At the heart of the OPA is an anaerobic membrane bioreactor – a hybrid technology that couples anaerobic digestion with membrane filtration. Developed through a collaboration between Kennedy’s Dr. Luke Roberson and the University of South Florida’s Dr. Daniel Yeh, the OPA was designed for an early planetary base scenario to help close the resource recovery loop, decreasing the agency’s dependence on resupply missions.
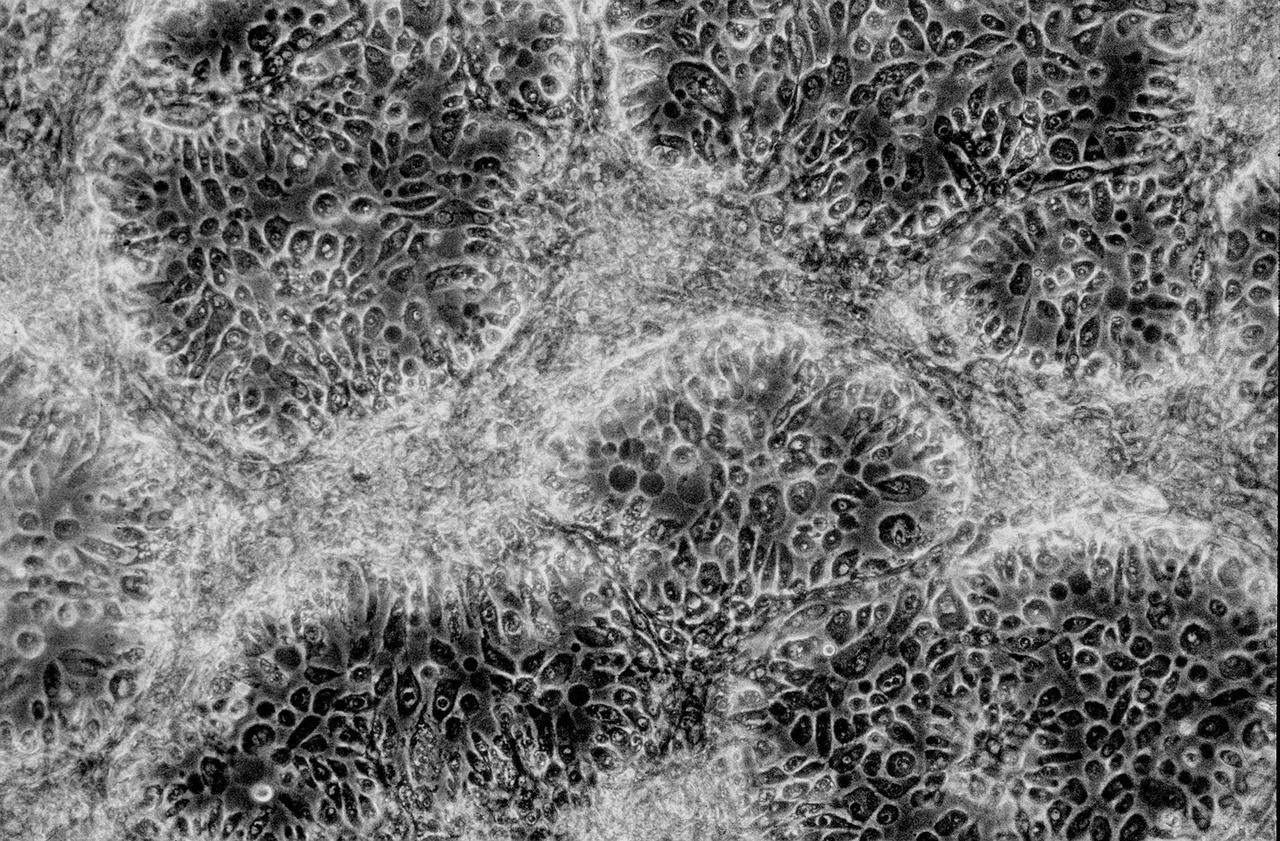
Isolation of human mammary epithelial cells (HMEC) from breast cancer susceptible tissue. Outgrowth of cells from duct element in upper right corner cultured in a standard dish; most cells spontaneously die during early cell divisions, but a few will establish long-term growth. NASA's Marshall Space Flight Center (MSFC) is sponsoring research with Bioreactors, rotating wall vessels designed to grow tissue samples in space, to understand how breast cancer works. This ground-based work studies the growth and assembly of human mammary epithelial cell (HMEC) from breast cancer susceptible tissue. Radiation can make the cells cancerous, thus allowing better comparisons of healthy vs. tunorous tissue. Credit: Dr. Robert Tichmond, NASA/Marshall Space Flight Center (MSFC).

STS-85 Payload Specialist Bjarni V. Tryggvason gives a thumbs up as he is assisted with his ascent/reentry flight suit in the Operations and Checkout (O&C) Building. He is a Canadian Space Agency astronaut and was born in Iceland. Tryggvason has also been a flight instructor for the Canadian Air Force. Tryggvason is the principal investigator of the Microgravity Vibration Isolation Mount now flying on the Russian Mir space station. During STS-85, Tryggvason will conduct vibration isolation mount and fluid physics investigations. His work to study how Shuttle vibrations affect the results of experiments will be valuable to the International Space Station program, since this experiment is planned for use on that space platform. Tryggvason will also conduct Bioreactor experiments and assist Mission Specialist Stephen K. Robinson with photography
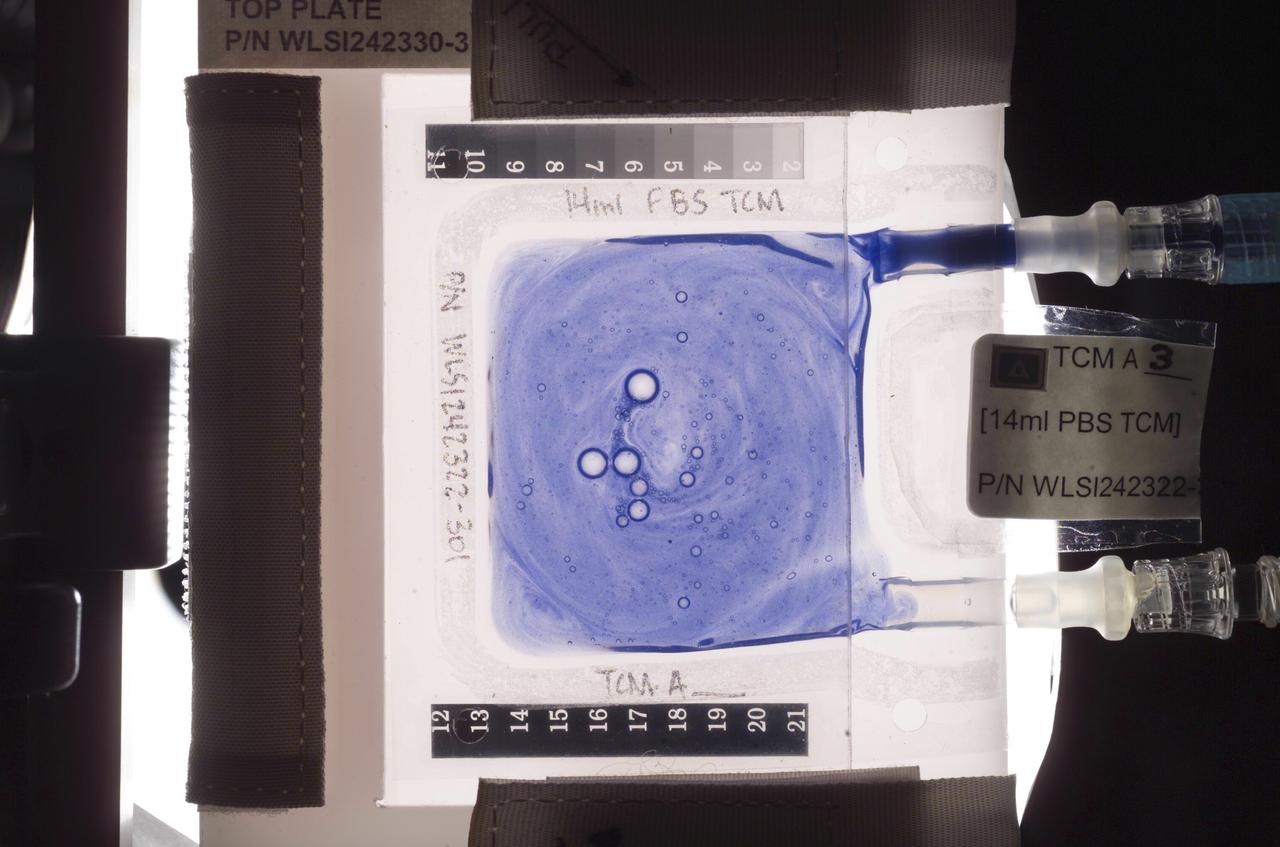
Aboard the International Space Station (ISS), the Tissue Culture Module (TCM) is the stationary bioreactor vessel in which cell cultures grow. However, for the Cellular Biotechnology Operations Support Systems-Fluid Dynamics Investigation (CBOSS-FDI), color polystyrene beads are used to measure the effectiveness of various mixing procedures. The beads are similar in size and density to human lymphoid cells. Uniform mixing is a crucial component of CBOSS experiments involving the immune response of human lymphoid cell suspensions. The goal is to develop procedures that are both convenient for the flight crew and are optimal in providing uniform and reproducible mixing of all components, including cells. The average bead density in a well mixed TCM will be uniform, with no bubbles, and it will be measured using the absorption of light. In this photograph, beads are trapped in the injection port, with bubbles forming shortly after injection.
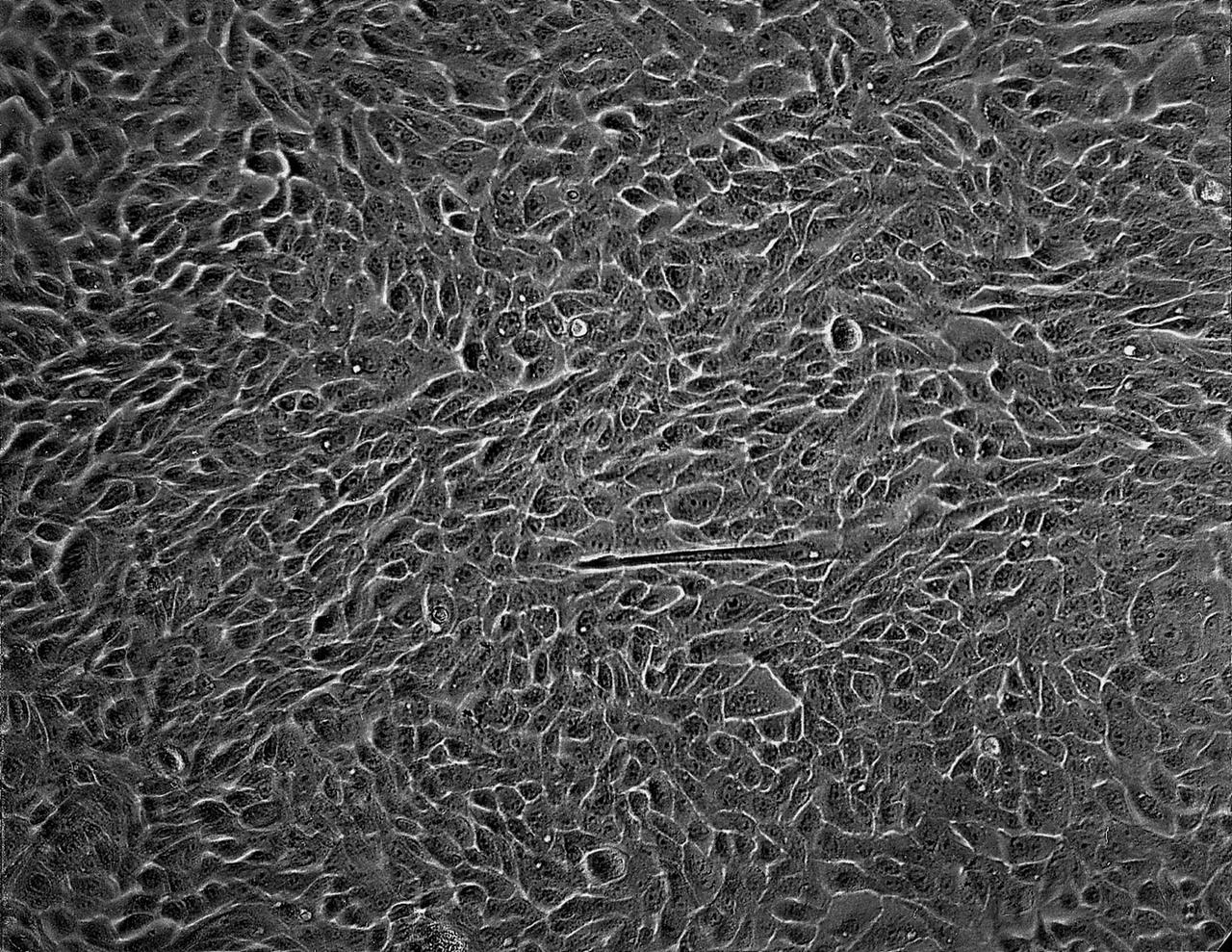
Isolation of human mammary epithelial cells (HMEC) from breast cancer susceptible tissue. Isolate of long-term growth human mammary epithelial cells (HMEC) from outgrowth of duct element; cells shown soon after isolation and early in culture in a dish. NASA's Marshall Space Flight Center (MSFC) is sponsoring research with Bioreactors, rotating wall vessels designed to grow tissue samples in space, to understand how breast cancer works. This ground-based work studies the growth and assembly of human mammary epithelial cell (HMEC) from breast cancer susceptible tissue. Radiation can make the cells cancerous, thus allowing better comparisons of healthy vs. tunorous tissue. Credit: Dr. Robert Tichmond, NASA/Marshall Space Flight Center (MSFC).
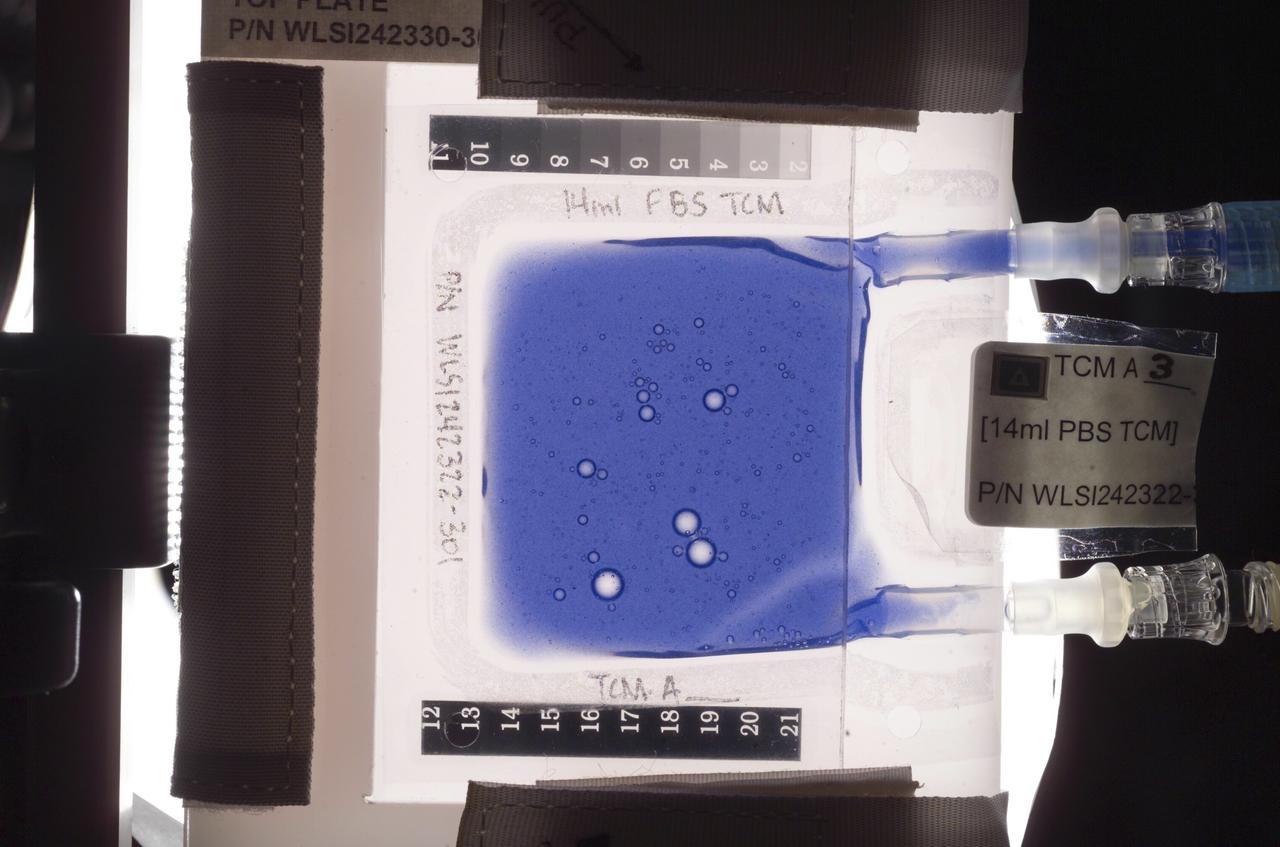
Aboard the International Space Station (ISS), the Tissue Culture Module (TCM) is the stationary bioreactor vessel in which cell cultures grow. However, for the Cellular Biotechnology Operations Support Systems-Fluid Dynamics Investigation (CBOSS-FDI), color polystyrene beads are used to measure the effectiveness of various mixing procedures. The beads are similar in size and density to human lymphoid cells. Uniform mixing is a crucial component of CBOSS experiments involving the immune response of human lymphoid cell suspensions. The goal is to develop procedures that are both convenient for the flight crew and are optimal in providing uniform and reproducible mixing of all components, including cells. The average bead density in a well mixed TCM will be uniform, with no bubbles, and it will be measured using the absorption of light. In this photograph, a TCM is shown after mixing protocols, and bubbles of various sizes can be seen.

Isolation of human mammary epithelial cells (HMEC) from breast cancer susceptible tissue. Same long-term growth human mammary epithelial cells (HMEC), but after 3 weeks in concinuous culture. Note attempts to reform duct elements, but this time in two dimensions in a dish rather that in three demensions in tissue. NASA's Marshall Space Flight Center (MSFC) is sponsoring research with Bioreactors, rotating wall vessels designed to grow tissue samples in space, to understand how breast cancer works. This ground-based work studies the growth and assembly of human mammary epithelial cell (HMEC) from breast cancer susceptible tissue. Radiation can make the cells cancerous, thus allowing better comparisons of healthy vs. tunorous tissue. Credit: Dr. Robert Tichmond, NASA/Marshall Space Flight Center (MSFC).
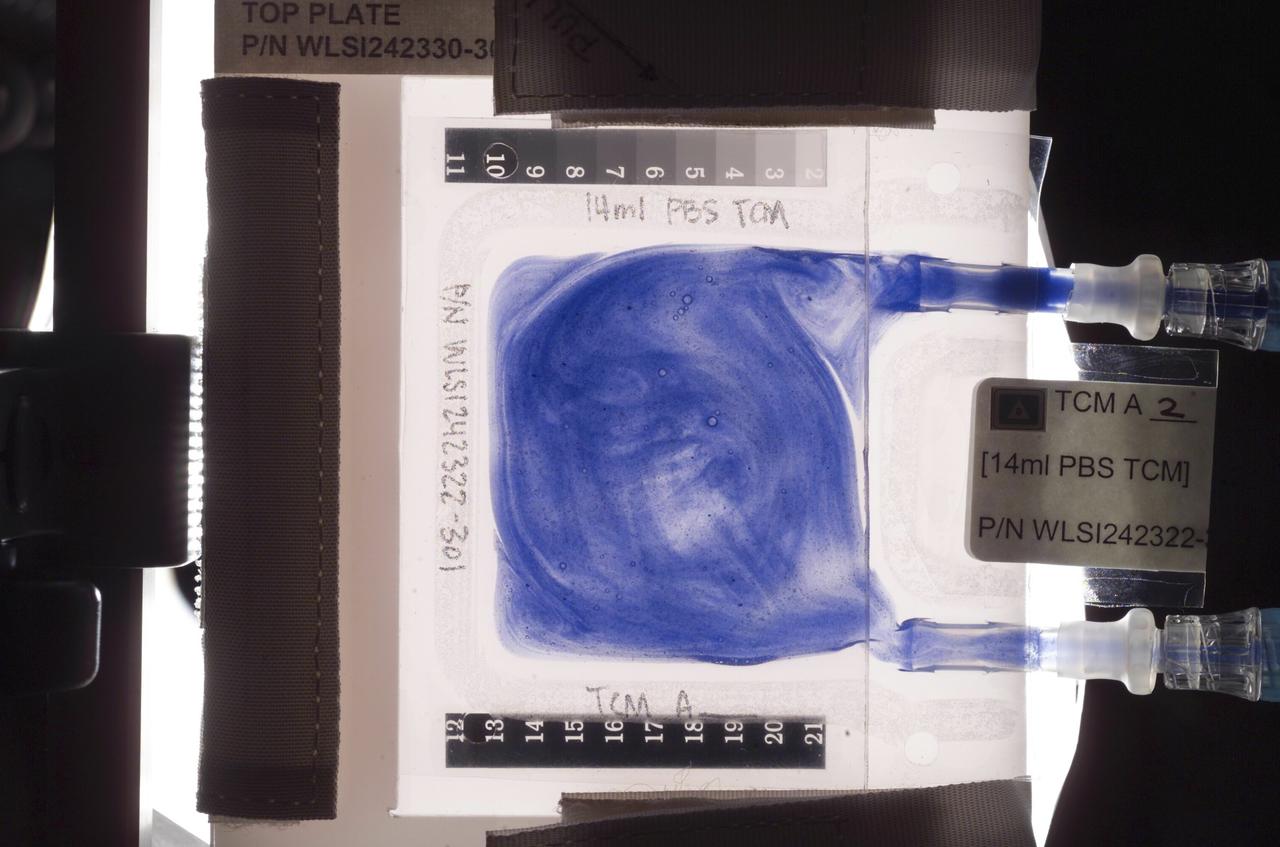
Aboard the International Space Station (ISS), the Tissue Culture Module (TCM) is the stationary bioreactor vessel in which cell cultures grow. However, for the Cellular Biotechnology Operations Support Systems-Fluid Dynamics Investigation (CBOSS-FDI), color polystyrene beads are used to measure the effectiveness of various mixing procedures. Uniform mixing is a crucial component of CBOSS experiments involving the immune response of human lymphoid cell suspensions. In this picture, the beads are trapped in the injection port shortly after injection. Swirls of beads indicate, event to the naked eye, the contents of the TCM are not fully mixed. The beads are similar in size and density to human lymphoid cells. The goal is to develop procedures that are both convenient for the flight crew and are optimal in providing uniform and reproducible mixing of all components, including cells. The average bead density in a well mixed TCM will be uniform, with no bubbles, and it will be measured using the absorption of light
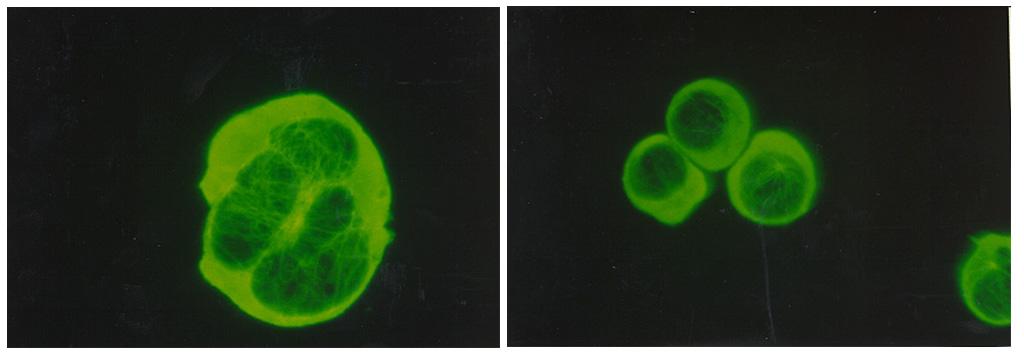
Biomedical research offers hope for a variety of medical problems, from diabetes to the replacement of damaged bone and tissues. Bioreactors, which are used to grow cells and tissue cultures, play a major role in such research and production efforts. The objective of the research was to define a way to differentiate between effects due to microgravity and those due to possible stress from non-optimal spaceflight conditions. These Jurkat cells, a human acute T-cell leukemia was obtained to evaluate three types of potential experimental stressors: a) Temperature elevation; b) Serum starvation; and c) Centrifugal force. The data from previous spaceflight experiments showed that actin filaments and cell shape are significantly different for the control. These normal cells serve as the baseline for future spaceflight experiments.

Isolation of human mammary epithelial cells (HMEC) from breast cancer susceptible tissue; A: Duct element recovered from breast tissue digest. B: Outgrowth of cells from duct element in upper right corner cultured in a standard dish; most cells spontaneousely die during early cell divisions, but a few will establish long-term growth. C: Isolate of long-term frowth HMEC from outgrowth of duct element; cells shown soon after isolation and in early full-cell contact growth in culture in a dish. D: same long-term growth HMEC, but after 3 weeks in late full-cell contact growth in a continuous culture in a dish. Note attempts to reform duct elements but this in two demensions in a dish rather than in three dimensions in tissue. NASA's Marshall Space Flight Center (MSFC) is sponsoring research with Bioreactors, rotating wall vessels designed to grow tissue samples in space, to understand how breast cancer works. This ground-based work studies the growth and assembly of human mammary epithelial cell (HMEC) from breast cancer susceptible tissue. Radiation can make the cells cancerous, thus allowing better comparisons of healthy vs. tunorous tissue. Credit: Dr. Robert Richmond, NASA/Marshall Space Flight Center (MSFC).